Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by its wide epidemiological, clinical, and immunological heterogeneity. The pathophysiology is associated with immunological, hormonal, genetic, and environmental factors1-3. Various studies have reported the effects of single nucleotide polymorphisms (SNPs) on the susceptibility to SLE4-6, including the vitamin D receptor (VDR) gene; however, its effects on VDR activity are still poorly understood7-9.
The VDR is the mediator of the biological activity of vitamin D. It is located in the nucleus of target cells, including immune cells (antigen-presenting cells, natural killer cells, B and T lymphocytes9-11. The VDR gene is located on chromosome 12q13.11 and regulates gene expression in various tissues that respond to vitamin D10,12.
In addition to the regulation of bone mineral homeostasis, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] is also involved in interleukin 2 (IL-2) inhibition, modulation of T-cell phenotypes13,14, the increase in the development of regulatory T- cells15,16, and the production of immunoglobulins17. 1,25(OH)2D3 inhibits interferon secretion and regulates the expression of IL-12 and nuclear factor kappa-B11,18. An in vitro study demonstrated that 1,25(OH)2D3 has a protective effect in autoimmune diseases19. Therefore, vitamin D3 is considered a regulator of the immune system11,20.
Several polymorphic markers of unknown functional consequence within the VDR gene have been studied for their potential role in SLE risk. There are four important polymorphisms of the VDR gene: TaqI (rs731236), located in exon 9; ApaI (rs7975232) in intron 8, BsmI (rs1544410) in intron 9, and FokI (rs10735810) at the initiation codon8,9,21-23.
However, the results of the association between these VDR gene polymorphisms and the risk of SLE are inconsistent in different studies carried out in various population groups, mainly due to different linkage disequilibrium coefficients and haplotype blocks in the populations, as well as to differences in sample size, population stratification, and variation in environmental factors between geographically separated areas8.
Population-based studies have shown that patients with SLE have significantly low serum levels of vitamin D18,24; like-wise, VDR gene polymorphisms are associated with higher activity/chronicity indices and an augmented risk of organic damage9,18,25.
This study aims to analyze the genetic association between VDR polymorphisms (TaqI, ApaI, BsmI, and FokI) and susceptibility to SLE, as well as its relationship with serum vitamin D levels in the Colombian Caribbean population.
Methods
A case-control study was conducted. A total of 133 patients diagnosed with SLE and 100 healthy individuals were included, in the adolescence (10-19 years), age group according to the World Health Organization (WHO), from the city of Barranquilla, on the Colombian Caribbean Coast. The diagnosis of SLE was performed by the presence of at least 4 of the 11 American College of Rheumatology (ACR) criteria26. Subjects were assessed both by specialized consultation and in a high-complexity level institution in the city of Barranquilla, Colombia.
Patients had no history of systemic diseases, other rheumatic disorders, infections, or malignancies. The control group with a negative ANA test had no history of autoimmune diseases or family relationships with patients with SLE. All patients and controls approved in writing by assent or informed consent depending on whether they were minors or adults in their voluntary participation in the study. This study was approved by the institutional ethics committee of the Universidad del Norte (Resolution No. 05).
Genomic DNA extraction
A team composed of phlebotomists, researchers, and physicians collected 10 ml of peripheral venous blood in Vacutainer® tubes, either with serum separator tube (SST) or with dipotassium EDTA (Beckton Dickinson & Company, Franklin Lakes, USA; Ref: 367856) for each participant, to obtain serum and the subsequent extraction of genomic DNA, respectively.
DNA was extracted from buffy coat (leukoplatelet fraction) of anticoagulated blood using the modified salting out protocol and precipitation with sodium perchlorate27. DNA concentration and quality were measured using the Nanodrop 2000/2000c spectrophotometer (Thermo Scientific, Waltham, MA, USA). Genomic DNA and serum samples were aliquoted and stored at -70°C until further analysis.
Serum vitamin D concentrations
Serum 25-hydroxyvitamin D concentrations were measured using enzyme immunoassay techniques: IDS-iSYS 25-Hydroxy Vitamin D (Immunodiagnosis Systems Ltd. Boldon, Tyne & Wear, United Kingdom). Reference values were established according to the Institute of Medicine (IOM) criteria, where <10ng/ml corresponds to deficient levels; 10-29 ng/ml is defined as insufficient vitamin D; ≥30- and <100 ng/ml are sufficient levels, and >100 ng/ml corresponds to possible vitamin D intoxication28.
SNP genotyping
The VDR gene polymorphisms (TaqI [rs731236 A/G], ApaI [rs7975232 A/C], FokI [rs2228570 A/G], and BsmI [rs1544410 C/T]) were genotyped by real-time PCR (RT-PCR), using commercially available kits: TaqMan® SNP Genotyping (Assay ID: C_16021387_20, C_2404008.10; C_28977635.10; C-12060045-20, and C—8716062.10; respectively [Applied Biosystems, FosterCity, CA, USA]).
Genotyping assays were performed on an ABI Prism 7300 RT-PCR equipment from Applied Biosystems, using a total volume per reaction of 5 µl (2.4 µl of DNA at an approximate concentration of 10ng/µl, 2.5 µl of Master Mix-2x, and 0.125 µl of Taqman Genotyping-40x probes specific to each SNP).
All PCR reactions were performed in duplicate. The cycling program consisted of an initial step of 10 min at 95 ° C, followed by 40 cycles from 15 s to 92 °C, and 1min at 60 °C per cycle. The genotypic assignment was performed automatically by applying Allelic Discrimination from the Applied Biosystems system, which considered an amplification quality ≥90% per sample.
Statistical analysis
Allelic, genotypic, and haplotypic frequencies were estimated in the study groups. In this study, non-probabilistic sampling was performed for convenience. Allele frequencies were used to estimate the Hardy-Weinberg genetic balance; in addition, the genetic association analysis with SLE was carried out at the allele level using the Arlequin v3.5 software.
The association analysis at the genotype level was performed using the Pearson x2 test, with Bonferroni correction of the p-value, in the statistical software SPSS v20 (IBM® SPSS® Statistics 20; IBM Corp., USA.). For these two analyses, the risk of SLE associated with each genotype or allele was also estimated, depending on the case, by calculating odds ratios (OR) and their corresponding 95% confidence intervals, using logistic regression models adjusted for the variable gender.
Association analyses between polymorphisms and serum vitamin D concentrations (quantitative data) were performed using the Mann-Whitney U and Kruskal Wallis tests, as appropriate, expressing mean values ± standard deviation (SD). On the other hand, the analyses with the categorical data of vitamin D were carried out using Pearson's x2 test or Fisher's exact test in cases with expected values <5, with Bonferroni correction of the p-value.
For the haplotype association analysis, the statistical package "Haplo.stats v1.6.8" (R version 3.0.2; http://www.r-project.org)29 was used; With this package, the Haplo.cc functions were used (logistic regression models for risk estimation: OR [95% CI], excluding haplotypes with frequencies <1%). In addition, 10,000 simulations were run to refine the p-value, represented as [psim]) and Haplo.glm (generalized linear regression model for estimating associations with numerical traits). Statistical significance was interpreted as p<0.05.
Results
In the sample studied, the average age was 16.19 ± 3.48 years; 94% (n = 125) were female; this was associated as a risk factor for SLE (OR: 10.8; 95% CI: 4.7-24.6).
The Hardy-Weinberg equilibrium distribution showed that the VDR polymorphisms: ApaI [rs7975232] and BsmI [rs1544410] were not found in genetic equilibrium for the control and case groups, respectively (p<0.05) (Table 1).
Table 1 Comparison of the genotypic and allelic frequencies of the vitamin D receptor polymorphisms in patients with systemic lupus erythematosus and the control group.
| VDR | Cases | H-W | Controls | H-W | OR | 95% CI | pa |
|---|---|---|---|---|---|---|---|
| (n = 133) | (n = 100) | ||||||
| FokI [rs2228570] | |||||||
| AA, n (%) | 20 (18) | 12 (12) | 2.12 | (0.9-4.8) | 0.081 | ||
| GA, n (%) | 51 (45.9) | 37 (37) | 1.75 | (0.9-3.17) | |||
| GG, n (%) | 40 (36) | 51 (51) | 0.47 | (0.20-1.07) | |||
| A, n (%) | 91 (41) | 1.00 | 61 (30.5) | 1.00 | 1.58 | (1.05-2.36) | 0.025* |
| G, n (%) | 131 (59) | 139 (69.5) | 0.63 | (0.42-0.94) | |||
| ApaI [rs7975232] | |||||||
| AA, n (%) | 25 (22.5) | 24 (24) | 0.61 | (0.2-1.4) | 0.317 | ||
| AC, n (%) | 59 (53.2) | 60 (60) | 0.58 | (0.2-1.1) | |||
| CC, n (%) | 27 (24.3) | 16 (16) | 1.62 | (0.7-3.7) | |||
| A, n (%) | 109 (49.1) | 1.00 | 108 (54) | 0.03* | 0.82 | (0.56-1.20) | 0.314 |
| C, n (%) | 113 (50.9) | 92 (46) | 1.21 | (0.83-1.78) | |||
| TaqI [rs731236] | |||||||
| AA, n (%) | 67 (60.4) | 56 (56) | 2.39 | (0.6-8.3) | 0.371 | ||
| GA, n (%) | 40 (36) | 36 (36) | 2.22 | (0.6-8.0) | |||
| GG, n (%) | 4 (3.6) | 8 (8) | 0.41 | (0.1-1.46) | |||
| A, n (%) | 174 (78.4) | 1.00 | 148 (74) | 1.00 | 1.27 | (0.81-1.99) | 0.291 |
| G, n (%) | 48 (21.6) | 52 (26) | 0.78 | (0.5-1.23) | |||
| BsmI [rs1544410] | |||||||
| CC, n (%) | 55 (49.5) | 55 (55) | 2.33 | (0.5-9.4) | 0.169 | ||
| CT, n (%) | 53 (47.7) | 38 (38) | 3.25 | (0.7-13.4) | |||
| TT, n (%) | 3 (2.7) | 7 (7) | 0.42 | (0.1-1.7) | |||
| C, n (%) | 163 (73.4) | 0.04* | 148 (74) | 1.00 | 0.97 | (0.62-1.49) | 0.893 |
| T, n (%) | 59 (26.6) | 52 (26) | 1.03 | (0.6-1.59) | |||
| H-W: Hardy-Weinberg equilibrium test, enhanced with 100,000 steps of the Markov chain. OR [95% CI]: logistic regression models. a x2 likelihood ratio. * p < 0.05 with Bonferroni correction. | |||||||
When comparing the genotypic and allele frequencies of the VDR polymorphisms in patients with SLE and the control group, a significantly higher frequency of the A allele of the FokI polymorphism [rs2228570] was found in cases than in controls (41 vs. 30%), associated with a 1.58 greater risk of SLE (OR: 1.58; 95% CI: 1.05-2.36; p = 0.025) (Table 1).
Haplotype analysis showed that, at the VDR locus, the ACCA haplotype of the TaqI/ApaI/BsmI/FokI polymorphisms was associated with a 2.28-fold increased risk of SLE (OR: 2.28; 95% CI: 1.12-4.66; p = 0.0) (Table 2).
Table 2 Haplotype analysis of vitamin D receptor polymorphisms in patients with systemic lupus erythematosus and control group.
| TaqI [rs731236] | ApaI [rs7975232] | BsmI [rs1544410] | FokI [rs2228570] | Controls (n = 100) (%) | Cases (n = 133) (%) | p a | dummy pb | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| A | A | C | G. | 0.23 | 0.158 | 0.052 | 0.058 | 0.90 | (0.4-1.7) |
| G. | A | T | G. | 0.13 | 0.107 | 0.114 | 0.107 | 0.95 | (0.4-2.2) |
| A | C. | C | G. | 0.298 | 0.215 | 0.135 | 0.137 | 1 | — |
| G. | A | T | A | 0.103 | 0.0726 | 0.666 | 0.669 | 0.94 | (0.4-2.2) |
| A | A | C | A | 0.0601 | 0.0642 | 0.593 | 0.600 | 1.28 | (0.4-3.6) |
| A | C | C | A | 0.14 | 0.259 | 0.00* | 0.00* | 2.28 | (1.12-4.66) |
| A | A | T | G | 0.0094 | 0.0575 | 0.00* | 0.00* | 14.7 | (0.2-767) |
|
a Empirical p- value. b Simulated p-value. c Reference value for the logistic regression model. * p < 0.05 with Bonferroni correction. | |||||||||
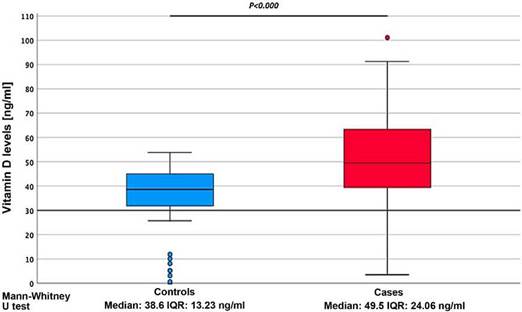
When analyzing the serum expression of vitamin D, it was found that the cases showed significantly higher serum concentrations, compared to the controls (49.5 ±24.06 ng/ml vs. 38.6 ±13.23 ng/ml) (p = 0.00) (Fig. 1). Vitamin D deficiency was evidenced in 11.3% of the patients (Fig. 2).
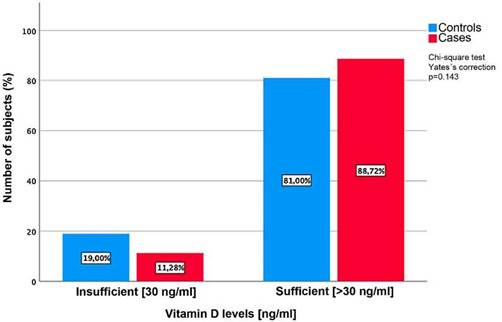
Figure 1 Comparison of serum vitamin D levels (ng/ml) between cases with systemic lupus erythematosus and controls.
Discussion
In the current study, a higher frequency of the A allele of the FokI polymorphism [rs2228570] was observed in the cases, which was associated with a greater risk of SLE. There was no statistical significance between the ApaI [rs7975232], TaqI [rs731236], and BsmI [rs1544410] polymorphisms and SLE in the codominant or allelic models. The ACCA haplotype of the TaqI, ApaI, BsmI, and FokI polymorphisms was associated with a higher risk of SLE. Additionally, individuals with SLE had significantly higher serum concentrations of vitamin D.
SLE is associated with the overproduction of cytokines. Shifts in the balance of Th1 and Th2 toward the overproduction of Th2 cytokines mediate hyperactivity of B cells, which promote plasma cell differentiation and antibody production30-32. The increase in the generation of autoantibodies and the deposition of immune complexes are the cornerstones of the clinical manifestations of SLE, such as lupus nephritis, cytopenias, and arthralgias33-35.
Vitamin D allows for greater tolerance by limiting the production of pro-inflammatory cytokines such as IL-12, which is a polarizing cytokine of T cells in Th1 cells and increases the production of anti-inflammatory cytokines such as IL-10. Consequently, it decreases autoreactivity and is one of the many mechanisms by which vitamin D can decrease the expression of autoimmune diseases9,10,36.
Abou-Raya et al.37 evaluated the effects of vitamin D supplementation on inflammatory cytokines (IL-1, IL-6 and TNFα), as well as the production of anti-dsDNA and anti-Sm in patients with SLE with vitamin D insufficiency. The levels of IL-1, IL-6, and TNFα decreased significantly, and the Systemic Lupus Erythematosus Activity Index (SLEDAI)38) scores improved in patients with SLE who received vitamin D supplementation, compared to the placebo group37. The immunomodulatory role of vitamin D is highlighted, demon-strating that deficiency is associated with higher rates of activity in autoimmune diseases, including SLE89,10,36.
The effects of vitamin D are mediated by the high affinity of the VDR which binds to the active form of vitamin D 1,25(OH)2D3 and is distributed in various tissues17,36. The VDR gene is highly polymorphic; however, the polymorphisms rs7975232 and rs7975232 in intron 8, rs731236 in exon 9, and rs2228570 in the start codon are the most studied variants39. In recent years, different studies have yielded conflicting results on the association between VDR gene polymorphisms and the risk of SLE9,10,23,40,41. Possible reasons for this disparity may be small sample sizes, low statistical power, or clinical heterogeneity8.
One meta-analysis included 11 studies with 1621 cases and 1883 controls that associated the BsmI, FokI, ApaI, or TaqI polymorphisms of the VDR gene with the risk of SLE41. An association between the BsmI B allele and the occurrence of SLE was observed in the general (OR: 1.726; 95% CI: 1.214-2.455) and Asian (OR: 1.952; 95% CI: 1.135-3.355) populations. The FokI T/C and TaqI polymorphisms were not associated with the risk of SLE in Caucasians. There was no significant association between the ApaI polymorphism and the risk of SLE for the general, Asian, and Caucasian populations41. In a study carried out by Monticielo et al.42 the VDR rs1544410 and rs2228570 polymorphisms were not associated with SLE in the European population, but higher concentrations of 1,25(OH)2D3 were found in patients carrying the rs2228570 ff42 genotype.
In our population, no significant associations were found between the BsmI, ApaI, and TaqI polymorphisms and the risk of SLE. However, greater susceptibility was shown through the FokI polymorphism in allelic models, only in agreement with series in the Arab population, such as those described by Imam et al.43 and Emerah and El-Shal40, with risks of susceptibility to SLE with OR: 1.617; 95% CI: 1.282-2.041; p < 0.000, and 1.948; 95% CI: 1.330-2.853; p < 0.001, respectively.
Emerah and El-Shal40 conducted a study to assess the VDR gene polymorphisms and haplotypes (ApaI, BsmI, and FokI) as risk factors or markers of SLE activity, finding that the ApaI AA genotype, the BsmI allele B, Bb, BB genotypes, FokI F allele, and VDR genotype FF were increased in the SLE group. In addition, serum levels of vitamin D 1,25(OH)2D3 were increased in SLE patients carrying the FokI ff genotype compared to subjects carrying the FF genotype.
In another meta-analysis (13 studies), Zhou et al.44observed the association between the rs1544410B and rs2228570f alleles and susceptibility to SLE in the general population, Asians, and Africans, but not in Caucasians. In Africans, the rs1544410 BB/bb, rs2228570 ff, and rs7975232 AA/aa genotypes were associated with SLE. However, there was no significant association between VDR rs1544410, rs2228570, rs7975232, and rs731236 and susceptibility to SLE in Caucasians.
Exposure to sunlight in SLE has a double effect, since ultraviolet B rays are essential for the cutaneous synthesis of vitamin D and, at the same time, is a possible trigger for a SLE flare45,46. Children with SLE are prone to vitamin D deficiency, as they avoid sunlight due to photosensitivity, use of sunscreen, physical inactivity secondary to pain or fatigue, and chronic use of medications such as corticosteroids and antimalarials that can improve vitamin D clearance47-51.
Hypovitaminosis D is associated with higher complement consumption and higher rates of disease activity. Ospina-Caicedo et al.24, in a cross-sectional study of 69 patients with SLE, found that 36.2% of them had low levels of vitamin D, which was higher than our study (11.3%); the lowest levels were found in patients with moderate to severe activity by SLEDAI score. In addition, higher consumption of complement proteins and high anti-dsDNA titers (p = 0.006) were evidenced. Patients who received steroids (0.5-1 mg/kg) had lower mean levels of vitamin D compared with those who did not (p = 0.048). Therefore, it is important to consider vitamin D supplementation in patients with SLE, especially when receiving corticosteroids.
Azab et al.51 showed that hypovitaminosis D is highly prevalent (57%) among Egyptian children and adolescents with SLE. Moreover, Yap et al.50 detected a vitamin D deficiency in more than a quarter of Australian adult individuals with SLE, with a high proportion of Caucasians in their cohort. A multicenter French study by Schoindre et al.49 reported similar findings, and the authors concluded that suboptimal vitamin D status was observed in more than 80% of SLE subjects. However, contrary to most reports, in our study higher levels of vitamin D were found in cases with SLE than in controls, which suggests that our population resides in areas that benefit from sufficient sunlight; in addition, it is also a signal of an adequate vitamin D supplementation scheme in corticotherapy; moreover, it could suggest a lower presence of anti-vitamin D antibodies between cases, which requires evaluation in future studies52.
In conclusion, the present study demonstrated the association between the VDR FokI rs2228570 polymorphism in allelic models and the ACCA haplotype of the TaqI, ApaI, BsmI, and FokI polymorphisms and an increased risk of SLE in the adolescent population. More studies are needed to assess the effects of these polymorphisms in SLE, especially their functional manifestations in other ethnic groups with larger sample sizes to confirm or refute our findings.
Limitations
To our knowledge, this is the first study that analyses VDR gene polymorphisms in adolescents with SLE from the Colombian Caribbean. However, some limitations in our study may affect the results. One of them was the failure to obtain clinical and immunological variables that would limit the establishment of associations between vitamin D levels, VDR gene polymorphisms, and SLE activity; furthermore, the sample size was relatively small. Because the results of the association between VDR polymorphisms and SLE have been different in different races and ethnic groups, more research is needed to distinguish such variables in our region.











 text in
text in