Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista MVZ Córdoba
versión impresa ISSN 0122-0268versión On-line ISSN 1909-0544
Rev.MVZ Cordoba v.15 n.2 Córdoba mayo/ago. 2010
ORIGINAL
Florfenicol concentrations in milk of lactating cows postreated by intramuscular or intramammary routes
Concentraciones de florfenicol en leche de vacas en lactancia postratamiento por vía intramuscular o intramamaria
1 Universidad de Antioquia, Facultad de Ciencias Agrarias, Grupo de investigación Centauro, Medellín, Colombia.
* Correspondencia: jdruiz@ces.edu.co
2 Universidad CES, Facultad de Medicina Veterinaria y Zootecnia.
3 Universidad de Antioquia, Facultad de Ciencias Exactas y Naturales, Grupo Interdisciplinario en Análisis de Residuos (GIAR), Medellín, Colombia.
Recibido: Septiembre 28 de 2009; Aceptado: Marzo 18 de 2010
ABSTRACT
Objective. To determine the florfenicol concentration in bovine milk after intramuscular or intramammary administration to establish the optimum withdrawal time, therapeutic efficacy, and its influence on milk yield. Materials and method. Twelve healthy lactating Holstein cows were selected from the University of Antioquia’s teaching dairy herd (Colombia), were randomly assigned to a control (n=6) group or florfenicol (n=6) group that received 20 mg/kg of florfenicol by intramammary and intramuscular routes, with a 15 days washout period between treatments. Results. The Tmax and Cmax for the intramuscular route were 6 hours and 2.86 mg/L respectively. The Tmax and Cmax for the intramammary route, were estimated at 0 hour and about 20000 mg/L respectively by extrapolated from regression line. The florfenicol elimination phase in milk had an average half-life of elimination (t1/2) of 19.8 hours and 4.9 hours for intramuscular and intramammary administration, respectively. The therapeutic efficacy only was reached by intramammary route, when minimal inhibitory concentration (M.I.C.) of florfenicol by Stahphylococcus aureus, was used as reference value. There was no statistically significant difference in milk yield between treated and non-treated cows. Conclusions. According to these results, post-treatment milk withdrawal should be no less than 3 days for intramammary administration, and at least 7 days for intramuscular administration. The therapeutic efficacy only was reached by intramammary route. In addition, there was no statistically significant difference in milk yield between treated and non-treated cows.
Key words: Pharmacokinetics, residues, tolerance, cows, florfenicol.
RESUMEN
Objetivo. Determinar la concentración de florfenicol en leche bovina después del tratamiento intramuscular o intramamario, para establecer el tiempo de retiro, la eficacia terapéutica, y su influencia sobre la producción láctea. Materiales y métodos. Se seleccionaron doce vacas Holstein en lactancia de la hacienda de Universidad de Antioquia (Colombia). Se asignaron al azar al grupo control (n=6) o al grupo florfenicol (n=6), al cual se le administró 20 mg/kg de florfenicol por vía intramamaria e intramuscular con 15 días de diferencia entre tratamientos. Resultados. La Tmax y Cmax para la vía intramuscular fueron 6 horas y 2.86 mg/L respectivamente. La Tmax y Cmax para la vía intramamaria, fueron estimados como 0 hora y aproximadamente 20000 mg/L respectivamente por extrapolación de la línea de regresión. La fase de eliminación del florfenicol en leche, tuvo una vida media de eliminación (t1/2) promedio de 19.8 horas y 4.9 horas para la vía intramuscular e intramamaria respectivamente. Cuando la concentración inhibitoria minima (C.I.M) del florfenicol para Stahphylococcus aureus, fue usada como el valor de referencia, la eficacia terapéutica sólo se alcanzó por la vía intramamaria. No existió diferencia estadística significativa entre las producciones lácteas de las vacas tratadas y no tratadas. Conclusiones. De acuerdo con estos resultados, el tiempo de retiro postratamiento no debe ser menor de 3 días para la administración intramamaria y al menos 7 días para la administración intramuscular. La eficacia terapéutica sólo se alcanzó por la vía intramamaria. Además no existió diferencia estadística significativa en la producción láctea de las vacas tratadas con respecto a las no tratadas.
Palabras clave: Farmacocinética, residuos, tolerancia, vacas, florfenicol.
INTRODUCTION
The presence of antibiotic residues in milk is an undesirable consequence of antibacterial treatments in lactating dairy cow(1). These residues could represent a threat for animal and public health, due to potential emergence of multidrug resistant bacteria (2,3).
The antibiotic florfenicol has entered the veterinary world market, with high efficacy for the treatment of bovine respiratory disease (4). It belongs to the same pharmacological group as chloramphenicol, which has been withdrawn from the market in many countries, while its use in others is limited because of the possibility of generating aplastic anemia in humans. Florfenicol (d-tre-2,2-dichloro-N-c-a- (fluoromethyl)-b-hydroxy-p-(phenethyl)acetamide), unlike chloramphenicol and tiamphenicol, has a fluorine atom instead of a hydroxyl group at C3, which makes it resistant to deactivation by transmissible plasmids of bacteria. It also has a methylsulphonyl group instead of the nitro group in chloranphenicol which is responsible for generating aplastic anemia in humans (5,6). Florfenicol has been approved for veterinary but not for human use (7).
Florfenicol, administered by intramuscular and intramammary routes, has good distribution in the bovine mammary gland (8,9). In addition, the bacterium Staphylococcus aureus has shown high susceptibility to phenicols (10,11) making florfenicol a good therapeutic option in S. aureus caused mastitis (12).
In a study carried out by Otero et al (12), it was shown that the volume distribution for florfenicol is low (Vd=0.47±0.1 L/Kg), in comparison to those of chloramphenicol in bovines, which suggests a greater molecular polarity due to replacing the nitro group by the methylsulphonyl. This value is similar to that reported by Soback et al (8) in 1995 (Vd=0.35±0.1 L/Kg), who administered florfenicol at a dose of 20 mg/Kg, indicating its distribution mainly in extracellular fluid. These results for volume distribution (Vd) contrast with the report by Varma et al (9) in 1986, and Adams et al (13) in 1987, who found 0.78 L/Kg (at a dose of 22 mg/Kg I.V.) and 0.872 L/Kg (at a dose of 11 mg/Kg I.V.) respectively (9,13). Another study in sheep in 2004 found Vd=0.55L/Kg (14). These Vds could indicate a good milk excretion.
The Non-observed Effect Levels (NOEL) for florfenicol based on reproductive studies in two generations was established at 1mg/Kg/day (15). For estimating the Admissible Daily Intake (ADI) a safety factor of 100 was used, which determined an ADI of 0.01 mg/Kg or 0.6 mg/person of 60 Kg of weight in average (16), or Theoretical Maximum Daily Intake (TMDI) (17). The TMDI divided by the consumption factor of the different animal tissues determines the Tol (Tolerance Levels) for the United States, and Maximum Residues Level (MRL) outside the United States (18). Although maximum permissible levels have not been established by the FDA for florfenicol, some studies suggest that the maximum provisional acceptable residues of florfenicol in milk could be approximately in a range between 0.08 and 0.2 mg/L, which means that a provisional acceptable tolerance level for florfenicol in milk, would be in a concentration range between 0.048 and 1.22 mg/L (17). This represents a possible target concentration for calculating extralabel withdrawal times for florfenicol in lactating cows.
The objective of the present work was to determine the concentrations of florfenicol in bovine milk after intramuscular or intramammary administration, to propose optimum withdrawal time for milk from treated cows, its possible therapeutic efficacy and its influence on milk yield.
MATERIALS AND METHODS
This work was done in compliance with the animal protection law 84 of 1989 for the Republic of Colombia.
Experimental population. Twelve healthy lactating Holstein cows were selected from the University of Antioquia teaching dairy herd located in the township of San Pedro de los Milagros, Colombia at 2.360 meters of altitude with an average temperature of 16°C. The cows were on pasture, grazing on Kikuyo (Pennisetum clandestinum) fields. A general clinical and mammary gland examination was conducted on the selected cows. Twelve lactating cows were randomly assigned to treatment protocol with florfenicol (n=6) with an interval of 15 days between intrammamary or intramuscular treatments, or to the control group (n=6).
Exclusion criteria. Dry cows (that are not in milking) and sick animals (through veterinary medical examination) were excluded from the trial. Cows having at least one of the following conditions were also excluded: first lactation, more than three parturitions, less than one month of lactation, more than seven months of lactation, abnormal mammary gland; and the presence of clinical (medical examination) or subclinical (according to CMT results) mastitis.
Clinical examination of the mammary gland. Mammary gland examination was conducted in order to detect abnormal mammary gland. It was assigned values according to following scale, 0=soft consistency, 1=any hard consistency, parenchyma alteration, inflammation or edema. Cows with the values different of zero were excluded.
Evaluation of subclinical mastitis (California Mastitis Test). Once the mammary gland was evaluated, milk was submitted to a semi-quantification of somatic cells using the CMT (California Mastitis Test) in order to exclude cows suffering subclinical mastitis. The CMT results were assessed in a scale of zero to five, according to reaction type. Each reaction type corresponds to an average cell count, within a probable ranking (19). Cows with the values different of zero were excluded.
Allocation to treatment groups. Once the cows were selected, after submitting them to the exclusion criteria, treatment 1 was carried out and, after 15 days of this treatment, they were subjected to treatment 2.
Treatment group 1. Six cows were subjected to an intramuscular application of florfenicol in a 30% solution (Nuflor® Schering Plough) at a dose of 20 mg/Kg of body weight, which is equivalent to 1 mL per each 15 Kg of body weight.
Treatment group 2. Fifteen days after treatment 1, the same six cows were subjected to an intramammary application of a 30% solution of florfenicol (NuflorÒ Schering Plough) at a dose of 20 mg/Kg of body weight, which is equivalent to 1 mL per each 15 Kg of weight. The total dose was divided in four aliquots that were administered in each one of the teats.
Control treatment. Six cows that fit the same criteria of selection as the cows of the experimental group were left without treatment, but daily milk yield (liters) was measured.
Procedure to obtain milk samples. The cows were milked in a milking room using a milking machine for 4 animals. Disinfection of each nipple was done before and after milking with a commercial iodine solution (povidone). The daily milk yield of each cow treated with florfenicol, and that of its respective control cow were appropriately registered from the day prior to the treatment and during 7 days following treatment.
Milk samples of cows treated with florfenicol were taken in each of the two groups at the following intervals: 0 hours and at 0.5, 1, 2, 3, 4, 6, 8, 24, 33, 48, 57, 72, 96, 120, 168, and 216 hours after intramuscular or intramammary administration. Approximately 6 ml of milk from each quarter were collected in the same glass bottles and transported refrigerated (at 4°C) to the laboratory for residue analysis. They were then stored at 0°C before processing within 24 hours.
Florfenicol assay. The analytical method proposed by Pfenning was used (20). A description of the extraction and validation of analytical method in our laboratory is further detailed elsewhere (21). The validation of the Gas Chromatography (GC) method was performed to determine florfenicol in bovine milk in a concentration range between 0.1 and 3.5 mg/L. The limit of detection of this assay was 0.1 mg/L. Precision, measured as the coefficient of variation (CV), showed results between 12 and 16% in the lower concentrations of the evaluated range; whereas in the high concentrations, the CV was lower than 6%. The accuracy and recovery showed that the lowest concentration level of florfenicol, 0.1 mg/L, had the highest recovery percentage (146.4%), and a CV of 13.4%. Concentrations higher than 0.1 and lower than 3.5 mg/L resulted in percentages of recovery that varied between 99 and 107% and CV between 4.92% and 8.71% (21).
Florfenicol quantification was conducted on each of the samples obtained.
Data analysis. An univariate descriptive analysis logarithmic regression and simple linear model as well as comparative tests of MANN WHITNEY and Pharmacokinetic analysis with the PK Solution software® were used for each treatment route.
The approximation at withdrawal time for milk in florfenicol treated cows was calculated, from
Where Dt is the time taken to go from Ci to Ct; Ct is tolerance concentration and is assumed to be 0.01 mg/L, since the maximum concentration of florfenicol allowed in food is zero. Some studies based on toxicological research have reported that a provisory tolerance florfenicol level in milk, should be in a concentration range between 0.048 and 1.22 mg/L (17). The Ci (starting concentration) is the florfenicol concentration in milk in intercept of elimination phase after intramuscular or intramammary administration.
T1/2 (half life of elimination): Time required to decrease the concentration of the drug in milk by one half and λz is the constant elimination rate (0.693/ T 1/2).
The results of the ANOVA analysis of one way of the dairy production of the six Holstein cows treated with florfenicol by intramuscular and intramammary routes and their respective controls were carried out with STATGRAPHICS version 4.1 software.
RESULTS
The average age and weight of the six cows in the study was 4.5 years (range: 3.33-5.42 years) and 575 kg (range: 480-615 kg), respectively. Cows were in their second and third lactation with an average of 163.5 lactation days (range: 130-210 days). Two of the cows had a pregnancy of less than 1 month and the four remaining cows were not pregnant. The average body score was 2.6 (range: 2.25-3.0; scale from 1 to 5). All of them presented a normal mammary gland and negative CMT test. The cows were pasturing on strips of Kikuyo prairie (Penisetum clandestinum) with water at libitum. The cows were milked at 7 a.m. and at 3 p.m. on the first treatment day, and at 7:00 a.m. and at 4:00 p.m. in the days following the treatment.
A control cow (without florfenicol treatment and in comparable reproductive and productive conditions) was assigned to each of the treated cows, of which florfenicol concentrations were measured at hour 0 (zero). These data served as external control, and guaranteed that there were no other florfenicol sources in the production lot apart from the treatment.
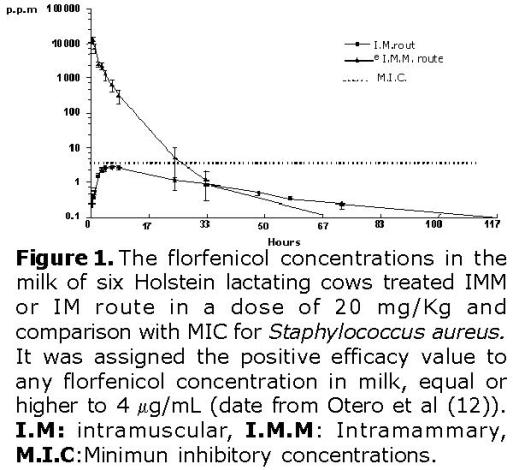
The profile of florfenicol concentrations in the milk of six Holstein dairy lactating cows treated by the intramuscular route at 20 mg/Kg is shown in figure 1. The florfenicol concentrations in milk after intramuscular administration are considered to fit a monocompartmental linear regression models, established in the program PK Solution®, according to the slope seen in figure 1.
A Cmax of 2.86 mg/L was attained 6 hours after intramuscular administration. The absorption phase is assumed up to that moment.
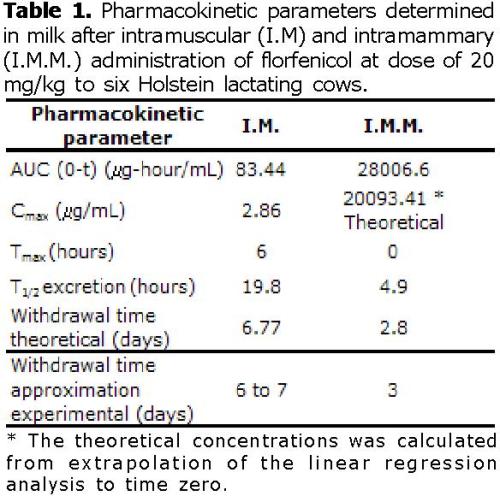
The florfenicol elimination phase in milk after florfenicol intramuscular administration (20 mg/Kg) was calculated between hours 8 and 120 post-administration as show in figure 1. The results from the regression analysis have a regression coefficient of 0.99, and an average half life of elimination (t1/2) of 19.8 hours, (Table 1); and an intercept of 2.98 mg/L.
Applying equation (1), and assuming that Ct: (0.01 mg/L), Ci: (intercept elimination phase 2.98 mg/L), T1/2 of 19.8 hours, resulted in a 162.57 hours (6.77 days) withdrawal time approximately, (Table 1).
The results calculated by a previous method were similar with dates experimental, because, after intramuscular administration the levels of florfenicol in the milk on the end day of study 7 were under of 0.1 mg/L (detection level).
The profile of florfenicol concentrations in the milk of the six cows, treated intramammary route at 20 mg/Kg is shown in figure 1. The florfenicol concentrations in milk after intramammary administration are considered to fit a three-compartmental nonlinear regression models, established in the program PK Solution®, according to the slope seen in figure 1.
The possible free passage of florfenicol from milk to systemic circulation can occur from minute 30 to minute 120 post-administration, as shown in figure 1. The pharmacokinetic regression analysis model gave a correlation coefficient of 0.99; an average absorption life of 21.15 minutes, and an intercept of 20093.41 μg/mL (concentration at zero minute). The intercept indicates the florfenicol concentration at 0 hour (zero).
The absorption-distribution phase corresponds to the time range between hours 3 and 8 post-administration as seen in figure 1. The pharmacokinetic regression analysis model gave a correlation coefficient of 0.98; an absorption half-life of 1.76 hours.
The elimination phase, takes place between hours 24 and 72, as seen in figure 1. As a result, the regression analysis indicated a correlation coefficient of 0.97; an average elimination half-life of 4.92 hours, and an intercept of 132.07 mg/L.
Applying equation (1), and assuming that Ct: (0.01 mg/L), Ci: (intercept elimination phase 132.07 mg/L), T1/2 of 4.92 hours, resulted in a 67.3 hours (2.8 days) withdrawal time.
The results calculated by the previous method are similar from the experimental data, in which it was established that the approximate withdrawal time of milk in cows treated with florfenicol by intramammary route, was 72 hours (3 days).
The results show, by comparison of the average and variances for the hypothesis test, that there are significant differences for florfenicol concentrations both between the intramuscular and intramammary routes of administration (p<0.01).
In this study florfenicol concentrations equal or higher to 4 μg/mL were considered effective, which is the minimum inhibitory concentration reported by Otero et al for the Staphylococcus aureus. Otero et al (12) evaluated 25 strains of aureus isolated from clinical and subclinical mastitis to determine MIC. For this, a range of concentrations of florfenicol from 0.125 and to 128 mg/ml was used. The florfenicol MIC for 24 strains in the study oscillated between 4 and 8 mg/mL which are considered normal for this kind of germ. These data are useful for evaluating the efficiency of florfenicol in the bovine mammary gland.
Thus, the positive efficiency value for any florfenicol concentration in milk was considered equal or higher to 4 μg/mL, and the variable efficiency was negative when the concentration was lower than the value mentioned (Figure 1).
Figure 1 shows that the only route of administration that reaches the efficiency level is the intramammary route and, specifically, during the time between zero (moment of administration) and 8 hours (prior to the first milking post-treatment). For the intramuscular treatment, the efficiency is considered negative since, from the first post-treatment hours, it had not reached concentrations equal or higher to the MIC (4 μg/mL) reported for the Staphylococcus aureus (12).
Concerning the production of milk in cattle lots treated with florfenicol by intramuscular route, an average dairy production of 13.6 liters for seven days during the evaluation and 13.98 liters for the control cows was obtained, without a statistically significant difference (p>0.05). Milk yield of the cows treated with florfenicol by the intramammary route was approximately 14.28 liters for the seven days of evaluation and 14.82 liters of milk for control cows during the same evaluation period with no statistically significant difference (p>0.05). This shows that there is no statistically significant difference in the milk production between the cows treated by either intramuscular or intramammary route and their respective controls (p>0.05).
DISCUSSION
In this work, the study of intramuscular administration of florfenicol demonstrated that after 30 minutes there are quantifiable florfenicol concentrations above 0.13 mg/L. These results contrast with those reported by Soback et al (8) in 1995, who, after 30 minutes, could not find florfenicol in quantifiable quantities in milk. This could be explained by the fact that his analytic technique allowed the quantification of a level of 0.5 mg/L florfenicol in milk, whereas in this study the quantifiable limit was 0.1 mg/L.
Regarding the maximum of florfenicol concentrations in milk after intramuscular treatment, these were reached at 6 hours with an average of 2.86 μg/mL, which does not differ statistically from the concentration of 2.75 μg/mL, obtained at 8 hours prior to the afternoon milking (first post-treatment milking). Soback et al (8) reported a maximal concentration of 1.6 μg/mL, obtained at 12 hours. In agreement with the study by Sobacks et al (8), our results show a common pharmacokinetic behavior pattern in which florfenicol is accumulated in the bovine mammary gland and reaches a maximum concentration in the milk just before the first post-intervention milking, which in this case was at 8 hours post-treatment, whereas it was at 12 hours in the study by Soback el al (8).
After the first milking, the concentrations of florfenicol decreased until an average of 1.213 µg/mL, 24 hours after intramuscular post-administration (before the second milking), which is equal to less than half of the level obtained before the first milking. This relation was also observed in the study by Soback et al (8), although in his case the average concentration was 0.6 μg/mL.
The levels of florfenicol in milk between 33 and 120 hours (5 days) after intramuscular treatment are lower than 1 μg/mL, as detectable by the analytical technique used. This establishes a non-useful therapeutic quantity for infections of treatment in the bovine mammary gland that is non regular regarding residues of this antibiotic in milk as established by the FDA.
Soback et al (8) maintains in his study, that after 48 hours of an intramuscular administration, florfenicol concentrations in milk are 0 μg/mL. However, the analytic technique used in this study allows quantification of lower concentrations than those detected by Soback et al (8). We consider this to be the reason for finding quantifiable levels of florfenicol in milk for a longer time period.
After treatment with florfenicol at a dose of 20 mg/kg by intramammary route, concentrations in milk at 30 minutes reached an average of 12.5±2.9 mg/mL. These data surpassed previous works by up to 12 fold the level reported by Soback et al (8). The results of this work can be explained if we take into account that average administration of florfenicol at 30% by quarter was 9.56 mL, which corresponds to a dose of 2.868 grams (2.868.000 mg) by quarter. It is inferred that at zero minute before the absorption process of florfenicol from the mammary gland towards the systemic blood system, concentrations of florfenicol were approximately 2009 3 mg/L (according to pharmacokinetics calculations). Assuming complete distribution of product in the volume of milk remaining after each milking, when the intramammary infusion was administrated, an estimated volume of approximately 570 ml milk was left in udder following the morning milkings.
It is observed from our results that 1 hour after the intramammary administration, there was a progressive decrease of florfenicol levels in milk, which is similar to the data obtained by Soback et al (8), although the values found in this study are higher than the ones described by him. At 8 hours prior to the first milking, an average florfenicol concentration in milk of 318.12 ± 129.62 mg/mL was found, while Soback et al (8) reported 202 ± 17g/mL at the same time.
Between 24 hours of administration of florfenicol by intramammary route to 57 hours, quantifiable levels of florfenicol are still found in milk, which differs from what was found by Soback et al (8), who found no florfenicol in milk at 48 hours, probably because the concentrations in milk were under the limit of detection allowed by his analytical method.
The progressive decrease in levels of florfenicol in milk after the intramammary administration may possibly be due to the following four factors: the absorption process from the mammary gland to the blood system, the dilution effect exerted by milk production, its elimination from the mammary gland by the excretion in milk, and its metabolism "in situ", for which, although the values are unknown, should not be discarded.
For intramuscular administration of florfenicol, the decrease of florfenicol in milk, after its distribution from the blood stream, may be due mainly to the re-absorption from the milk to the blood stream, the excretion in milk itself and the metabolism "in situ" into udder.
The analysis of the results poses questions concerning two essential parameters used to evaluate the performance of an antibiotic in the mammary gland: first, the prediction of the therapeutic efficiency and second, the residual potential of any antibiotic that reaches the mammary gland of cattle in the process lactating.
Concerning the therapeutic efficiency, the fundamental parameter for evaluation of this aspect is the Minimum Inhibitory Concentration (MIC) of the microorganism or microorganisms to be controlled. As was mentioned before, the most common isolated germs of the bovine mammary gland in several countries are Streptococcus and Staphylococcus sp (22). Streptococcus agalactiae is the most prevalent germ, which shows good sensitivity to b-lactamic antibiotics (23). Staphylococcus aureus is the hardest bacteria to treat, due to its resistances to antibiotics (22,24). Therefore, in milk farms it is important to apply new pharmaco-therapeutic alternatives which show efficiency in the treatment for aureus.
Concentrations of florfenicol in milk after a 20 mg/Kg intramuscular treatment are sub-optimum for bacteria such as aureus isolated from bovine mastitis, thus this route of administration is not recommended for treatment of mastitis. However middle strains should be evaluated and it would be better to determine the MIC in milk, which is the medium in which the antibiotic would act. This conclusion is in contrast to that drawn by Otero et al (12), who considered the plasmatic concentrations obtained by florfenicol through the intramuscular route to be efficient. However, they concluded that the best parameter to evaluate efficiency in this type of pathologies were the concentrations of florfenicol in milk.
For the intramammary route, concentrations are maintained above 4 μg/ml from 0 minute to 480 minute after treatment, thus its effect is expected all along that period. Soback et al obtained a similar pharmacokinetic behavior, showing that 20 mg/Kg of body weight of florfenicol by intramammary route reached concentrations above 4 μg/ml in milk from the time of administration until 11 hours post-treatment.
Concerning the residues of florfenicol in milk, the FDA (Foods and Drug Administration) establishes that any quantity of florfenicol in milk is considered as violative (7). Some studies based on toxicological investigations, have reported that a provisional tolerance level of florfenicol in milk, would be in the range of concentration between 0.048 and 1.22 mg/L (17).
If we assume that the concentration of 0.01 mg/L is the maximum allowed level of residues of florfenicol in milk according to suggestions of Baynes et al (17) withdrawal-time after intramuscular treatment, should be probably 6.77 days and for the intramammary treatment 2.8 days. These withdrawal-times could be lower if a greater level of tolerance of 0.10 mg/L is established.
In conclusion the intramammary and intramuscular florfenicol treatment in lactating cows using 20 mg/Kg, produce residues in milk.
Regarding the intramammary treatment, up to concentrations 4 mg/mL are achieved by its applications to the next milking (8 hours) using a dose of 20 mg/Kg divided into every quarter. Besides, persistency of residues in milk using the intramammary treatment is shorter by 4 days compared to the case of the intramuscular treatment.
In this study the milk yield of treated groups with florfenicol, as compared to their controls without treatment, remained unaltered.
Some recommendations, it is necessary to undertake research on the residues and persistency of diverse antibiotics in biological fluids of livestock animals as a basis for the pharmaco-epidemiologic follow-up of public health problems that are both serious and resistant, and the new re-emergence of infections.
The efficiency of florfenicol can be determined by theoretically pharmacokinetic models. Therefore, is necessary to carry out prior clinical trials.
In conclusion, according to these results, post-treatment milk withdrawal should be no less than 3 days for intramammary administration, and at least 7 days for intramuscular administration. The therapeutic efficacy only was reached by intramammary route. In addition there was no statistically significant difference in milk yield between treated and non-treated cows.
REFERENCES
1. Fedorka-Cray PJ, Dargatz DA, Wells SJ, Wineland NE, Miller MA, Tollefson L, et al. Impact of antimicrobic use in veterinary medicine. J Am Vet Med Assoc 1998; 213(12):1739-41. [ Links ]
2. Elviss NC, Williams LK, Jørgensen F, Chisholm SA, Lawson AJ, Swift C, et al. Amoxicillin therapy of poultry flocks: effect upon the selection of amoxicillin-resistant commensal Campylobacter spp. J Antimicrob Chemother 2009; 64(4):702-11. [ Links ]
3. Cox LA Jr, Popken DA, Mathers JJ. Human health risk assessment of penicillin/aminopenicillin resistance in enterococci due to penicillin use in food animals. Risk Anal 2009; 29(6):796-805. [ Links ]
4. Hoar BR, Jelinski MD, Ribble CS, Janzen ED, Johnson JC. A comparison of the clinical field efficacy and safety of florfenicol and tilmicosin for the treatment of undifferentiated bovine respiratory disease of cattle in western Canada. Can Vet J 1998; 39(3):161-6. [ Links ]
5. Lemos M. Antimicrobianos que inhiben la síntesis de proteínas. En: Botana L, Landoni F, Martín-Jiménez T, editores. Farmacología y terapéutica veterinaria. España: McGraw-Hill Interamericana. 2002. [ Links ]
6. Turton JA, Havard AC, Robinson S, Holt DE, Andrews CM, Fagg R, et al. An assessment of chloramphenicol and thiamphenicol in the induction of aplastic anaemia in the BALB/c mouse. Food Chem Toxicol 2000; 38(10):925-38. [ Links ]
7. Smucker J (Milk Safety Branch, Food and Drug Administration). Milk and Milk Product Equipment - A Guide for Evaluating Construction. Washington DC. 2000. [ Links ]
8. Soback S, Paape MJ, Filep R, Varma KJ. Florfenicol pharmacokinetics in lactating cows after intravenous, intramuscular and intramammary administration. J Vet Pharmacol Ther 1995; 18(6):413-7. [ Links ]
9. Varma KJ, Adams PE, Powers TE, Powers JD, Lamendola JF. Pharmacokinetics of florfenicol in veal calves. J Vet Pharmacol Ther 1986; 9(4):412-25. [ Links ]
10. Drago L, De Vecchi E, Fassina MC, Mombelli B, Tocalli L, Gismondo MR. Comparative in vitro activity of thiamphenicol-glycinate and thiamphenicol - glycinate - acetylcysteinate and other antimicrobials against respiratory pathogens. Arzneimittelforschung 2001; 51(4):315-24. [ Links ]
11. Neu HC, Fu KP. In vitro activity of chloramphenicol and thiamphenicol analogs. Antimicrob Agents Chemother 1980; 18(2):311-6. [ Links ]
12. Otero PE, Rebuelto M, Albarellos G, Kreil V, Ambros L, Denamiel G, et al. Farmacocinética del florfenicol en bovinos en lactación. In Vet 1999; 1:33-38. [ Links ]
13. Adams PE, Varma KJ, Powers TE, Lamendola JF. Tissue concentrations and pharmacokinetics of florfenicol in male veal calves given repeated doses. Am J Vet Res 1987; 48(12):1725-32. [ Links ]
14. Lane VM, Villarroel A, Wetzlich SE, Clifford A, Taylor I, Craigmill AL. Intravenous and subcutaneous pharmacokinetics of florfenicol in sheep. J Vet Pharmacol Ther 2004; 27(4):191-6. [ Links ]
15. Center of Veterinary Medicine and FDA. General principles for evaluating the safety of compounds used in food-producing animals. U.S. Department of Health and Human Services. Washington D.C: United States Food and Drug Administration; 1994. [ Links ]
16. Schering Plough Animal Health Group (Food and Drug Administration) Freedom of information FOI summary. Supplemental new animal drug application. Nuflor injectable solution (florfenicol). Document NADA; 2006. [ Links ]
17. Baynes RE, Martín-Jiménez T, Craigmill AL, Riviere JE. Estimating provisional acceptable residues for extralabel drug use in livestock. Regul Toxicol Pharmacol 1999; 29(3):287-99. [ Links ]
18. Riviere JE, Webb AI, Craigmill AL. Primer on estimating withdrawal times after extralabel drug use. J Am Vet Med Assoc 1998; 213(7):966-8. [ Links ]
19. Saloniemi H. Use of somatic cell count in udder health work. In bovine udder and mastitis. University of Helsinki. 1995. [ Links ]
20. Pfenning AP, Madson MR, Roybal JE, Turnipseed SB, Gonzales SA, Hurlbut JA, et al. Simultaneous determination of chloramphenicol, florfenicol, and thiamphenicol residues in milk by gas chromatography with electron capture detection. J AOAC Int 1998; 81(4):714-20. [ Links ]
21. Ruiz JD, Zapata MM, López C. Validación de un método analítico para la determinación de florfenicol en leche bovina. Revista Vitae 2003. 10:27-35. [ Links ]
22. Ramírez N, Gaviria G, Arroyave O, Sierra B, Benjumea J. Prevalencia de mastitis bovina en vacas lecheras lactantes en el municipio de San Pedro de los Milagros, Antioquia. Rev Col Cienc Pec 2000. 14:76-87. [ Links ]
23. Ruiz JD, Ramírez N, Arroyave O. Determinación de concentraciones inhibitorias mínimas a algunos antibióticos de las bacterias aisladas de glándula mamaria bovina en San Pedro de los Milagros. Antioquia. Rev Col Cienc Pec 2001; 14:141-152. [ Links ]
24. Londoño ME, Sierra CA. Caracterización de la información clínica del laboratorio de microbiología de la facultad de Medicina Veterinaria y de Zootecnia de la Universidad de Antioquia [trabajo de grado]. Medellin, Colombia: Universidad de Antioquia, Facultad de Medicina Veterinaria y de Zootecnia; 1998. [ Links ]