Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268On-line version ISSN 1909-0544
Rev.MVZ Cordoba vol.16 no.1 Córdoba Jan./May 2011
ORIGINAL
Distribution of ectoparasites of Canis lupus familiaris L. (Carnivora: Canidae) from Panama
Distribución de los ectoparásitos de Canis lupus familiaris L. (Carnivora: Canidae) de Panamá
1Instituto Conmemorativo Gorgas de Estudios de la Salud, Ciudad de Panamá, Panamá
*Correspondencia: bermudezsec@gmail.com
Recibido: Junio de 2010; Aceptado: Diciembre de 2010
Abstract
Objetive. To determine the distribution of ectoparasites in dogs in Panama. Materials and methods. There were surveyed 720 canines belonging to 57 communities. Results. The results showed that 84% of the dogs were infested with at least one species of ectoparasite. Dogs from lowlands showed a higher percentage of parasitism and a greater biodiversity of parasites than dogs from highlands. There were found seven species of ticks, four species of fleas, two species of lice, and one specie of botfly. The ticks Rhipicephalus sanguineus, Amblyomma cajennense, A. ovale and the flea Ctenocephalides felis were widespread; however Ixodes boliviensis and Pulex simulans showed a much narrower geographic distribution and they were found only in dogs from highlands. The flea species Rhopalopsyllus cacicus and the tick Haemaphysalis juxtakochi were found for the first time in panamanian dogs. Conclusions. The environmental situation in Panama, can encourage that wildlife ectoparasites parasitized dogs in absence of their native hosts. This condition may increase transmission risk of some diseases where the ticks and fleas are vectors.
Key words: Dogs, ectoparasites, Panama. (Source: DeCS, ICYT, AIMS).
Resumen
Objetivo. Determinar la distribución de ectoparásitos de perros en Panamá. Materiales y métodos. Se examinaron 720 individuos en 57 comunidades. Resultados. Los resultados demostraron que el 84% de los perros presentaron al menos una especie de ectoparásito. Los perros de tierras bajas mostraron un mayor porcentaje de parasitismo y mayor biodiversidad de parásitos que los animales de tierras altas. Se encontraron siete especies de garrapatas, cuatro de pulgas, dos de piojos y una de mosca. Las garrapatas Rhipicephalus sanguineus, Amblyomma cajennense, A. ovale y la pulga Ctenocephalides felis mantuvieron una distribución más amplia; mientras que Ixodes boliviensis y Pulex simulans sólo se reportaron en tierras altas. La pulga Rhopalopsyllus cacicus y la garrapata Haemaphysalis juxtakochi se reportaron por primera vez en perros de Panamá. Conclusiones. La situación medioambiental en Panamá puede propiciar que la fauna de ectoparásitos parasiten perros ante la ausencia de hospederos nativos, esta condición puede aumentar el riesgo de transmisión de algunas enfermedades en las que las garrapatas y pulgas son vectores.
Palabras clave: Perros, ectoparásitos, Panamá. (Fuente: DeCS, ICYT, AIMS).
Introduction
Dogs were the first animal species to be domesticated by humans and have been used extensively as hunters, protection purposes and as food (1, 2). The domestication process took place in several isolated geographic localities over a period of many centuries, producing the diversity of breeds that we know today (3). Presently, dogs are considered pets instead of wild animals, and live in close association with humans.
The domestication of the dog also created new ecological interactions between the ectoparasites of these canines and humans, exposing people to new pathogenic agents. There are many ectoparasites of dogs that serve as reservoirs, vectors, or intermediate hosts for pathogenic bacteria, fungi and metazoan parasites (e.g., tapeworms and roundworms). Bacteria such as Rickettsia rickettsii, Rickettsia felis, Ehrlichia chaffeensis and parasitic helminths like Dipylidium caninum and Hymelonepis nana, are examples of microorganisms that are associated with ectoparasites of dogs and that also can affect humans (4).
Studies of ectoparasites of panamanian dogs are scarce. The only complete checklist was published in 1966 in the "Ectoparasites of Panama", by Fairchild et al (5) and there have been no other published studies on this subject. The objective of this paper is to present new data regarding the distribution of the ectoparasites infesting Panamanian dogs and to describe their ecological relationships.
Materials and methods
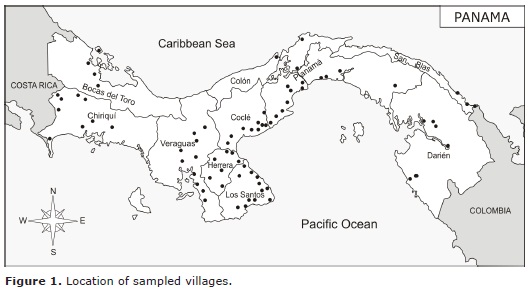
Study site. From June 2007-April 2009, we collected ectoparasites from dogs representing 57 communities in Panama (Figura 1) as part of a larger effort from several different research projects (see acknowledgement). The selection of dogs depended on the owners consent.
Conservation of ectoparasites. The ectoparasites were preserved in 95% alcohol. Engorged ticks nymphs were collected and kept alive in plastic bottles plugged with cotton, and then placed in an incubator (average temperature of 29°C and 80% of humity) until molt.
Identification of ectoparasites. For the identification we using published descriptions for ticks (5, 6), lice (7), fleas (8). In addition, we revised the reference material from the Colección Zoológica "Dr. Eustorgio Méndez" of the Gorgas Memorial Institute for Health Research (CoZEM-ICGES for Spanish acronomy) in Panama City. The ectoparasites were placed in CoZEM-ICGES collection.
Results
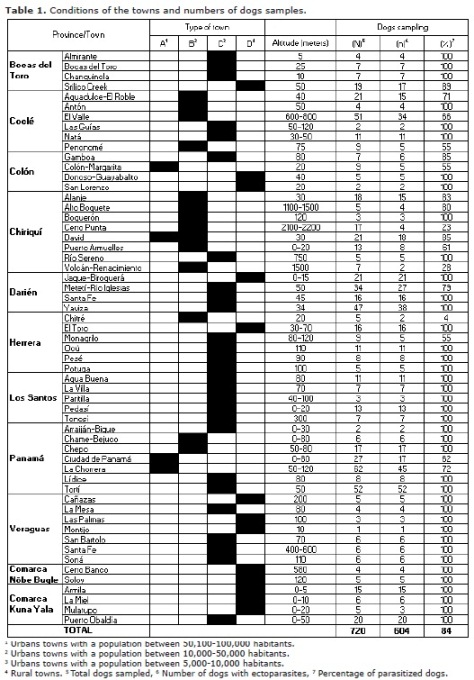
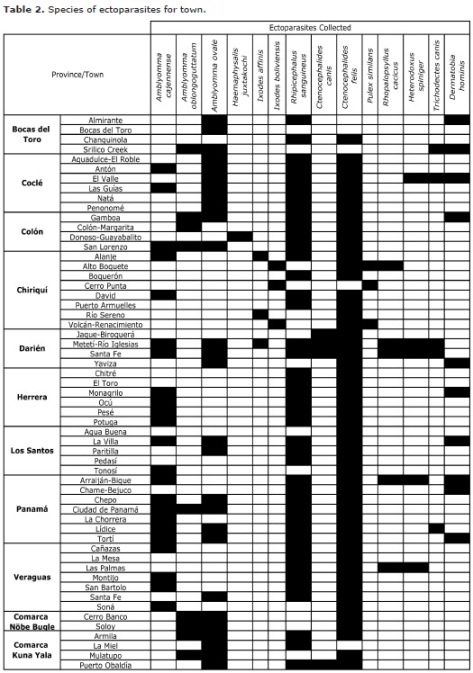
There were examined 720 dogs from 57 towns and found that 84% of the animals were infested by at least one ectoparasite (Table 1). The highest prevalence of parasitism was observed in dogs from suburban and rural localities in lowlands (altitude: 0-1000 meters). Dogs from highland towns presented a much lower prevalence. Dogs from lowland towns exhibited a greater richness of ectoparasites than conspecifics from the highlands (Table 2).
Seven species of ticks, four species of fleas, two species of lice and one botfly were observed (Table 2). The only species that it could raise was Amblyomma cajennense. The species with greatest distributions were the flea Ctenocephalides felis and the ticks Rhipicephalus sanguineus s.l., A. cajennense and Amblyomma ovale. The tick, Ixodes boliviensis and the fleas Pulex simulans and Rhopalopsyllus cacicus, were restricted to high-lands.
Discussion
Rhipicephalus sanguineus s.l. was found in all towns from rural and urban lowlands. This species was introduced to the New World from Old World dogs and infest multiple species of Carnivores with domestic cats and dogs being the preferred hosts (9). According to Guglielmone et al (9), the development of this tick, including an extra-parasite cycle after feeding, enables it to spread to new localities and infest new host, including humans.
This close proximity to humans makes R. sanguineus the most implicated pathogens in diseases dispersions, such as spotted fever on America (R. rickettsii) (10,11), mediterranean spotted fever (Rickettsia conorii) (12) and canine ehrlichiosis (Ehrlichia canis) (13). In Panama, genetic material of R. amblyommii has been found in R. sanguineus (14), species implicated to cause a mild fever, even when its impact to humans is unknown in many counties.
In this study, we found the co-existence of R. sanguineus with A. cajennense, A. oblongoguttatum, A. ovale, Haemaphysalis juxtakochi and Ixodes affinis on the same host or localities. The frequency of co-existence of R. sanguineus and A. cajennense on the same dogs was associated with horses and cattle in areas of pecuariam activities. In contrast to R. sanguineus, the immature and adults of A. cajennense infests a wide variety of host and is one of the most commons ticks species found on domesticated animals in Panama (5). This species show a preference for disturbed areas, especially sites where deforestation creates habitats that are more adequate for their establishment (15).
In Latin American, A. cajennense affects mostly humans and transmits R. ricketsii in many countries (16). In Panama, A. cajennense has been found as vector of R. rickettsii (17) and R. amblyommii was detected from the genetic material of horses and dogs (14). In this study, nymphs and adults of A. cajennense were collected in dogs.
The co-existence of R. sanguineus with A. oblongoguttatum, A. ovale, H. juxtakochi and I. affinis occurred in rural populations, indigenous towns and in sub-urban areas near forests. Dogs in communities close to forest were often used for hunting wild animals and this function may explain the infestations of dogs by these ticks. Immature stages from these species parasitize mostly small mammals and birds while adults infest medium to large-sized mammals, including dogs (5, 9). The tick parasitism on domestic animals could allow alternate conditions for the establishment of new pathogens in humans populations increasing the associated risks for pathogen transmission. This is the first record of H. juxtakochi parasitized panamanian dogs. Former records of this species include host as Nasua nasua, tapirs, deer and the porcupine Coendou rothschildi (5, 9).
Ixodes boliviensis was only found in rural communities within an elevation of 1100-1500 meters. Fairchild et al (5) stated that this species was most common in dogs from altitudes close to 850 meters (2500 feet); however, during this study, we found did not find any I. boliviensis in localities under this altitude. Instead, we observed R. sanguineus and A. ovale in towns with similar altitudes to those cited, as habits for I. boliviensis, by Fairchild et al (5). Differences between these studies can be explained by the increases in human populations in those communities that have created conditions favorable for the establishment of R. sanguineus.
In contrast to highlands communities as Boquete, Volcan and Cerro Punta (Table 2), which have also experienced a significant increase in human populations, only I. boliviensis has become established, suggesting that towns in altitudes greater than 1000 meters limit the distribution of R. sanguineus in Panama. Even though the possibility of infested dogs with R. sanguineus from lowlands can occur in these populations, the establishment of population of these ticks needs further verification.
The Costa Rican localities from the Province of Cartago (Puricil and Tapanti) have altitudes between 1300 and 1400 meters. In these areas, I. boliviensis is present but not R. sanguineus, a result that has also been observed in Panama (Carlos Víquez, personal communication). In urban areas from the Costa Rican cities like Heredia and San José, which has an average altitude of 1200 meters, R. sanguineus is commonly found, while I. boliviensis only is observed in rural zones (Grace Alpízar, personal communication).
These differences in the distribution between R. sanguineus and I. boliviensis in Costa Rica and Panama at similar altitudes, but different human population densities, can be explained by the extension of urban development. A city with wide urban zones provides more opportunities for R. sanguineus to colonize and develop populations. Similarly, these conditions minimize opportunities for the establishment of I. boliviensis, due this species needs different hosts for immature stages as well as adequate oviposition sites. Additionally, high levels of urbanization increase the local temperature and influences general weather patterns; conditions that also favor the establishment of R. sanguineus.
Ctenocephalides felis maintain a wide distribution across Panama, it was found on every dog from urban, suburban and rural localities within 0-1400 meters. A previous study showed that C. felis is the fleas with a major dispersion in Panama (8). In contrast, C. canis has a narrower range, being only found in rural localities from Darién and Kuna Yala (Table 2). This flea is considered to be rare species and was included in the Tipton and Méndez (8) based upon only one reference point by Dunn (op. cit).
Pulex similans were captured exclusively on dogs from Boquete and co-exists with I. boliviensis, C. felis and R. cacicus saeus. Tipton and Méndez (8) discussed the difference between P. irritans and P. simulans, and characterized P. simulans as a lowland species and P. irritans as highland species (over 5000 feet). To distinguish these species, we used the aedeagus, the main morphological character proposed by Smit (18). We reviewed this character in specimens of P. irritans from United States and Colombia (CoZEM), compared them with the specimens from Volcan and Boquete, and found that they exhibited the aedeagus morphology of P. simulans.
Rhopalopsyllus cacicus was only found in dogs from Boquete. Tipton and Méndez (8), affirm that R. cacicus is a parasite of several species of mammals, such as opossums (Metachirus nudicaudatus and Philander opossum), armadillos (Dasypus novencinctus), rodents (Proechimys semispinosus, Agouti paca and Dasyprocta punctata) and carnivores (Nasua nasua). Our observation represents the first geographical record of this ectoparasite for Panamanian dogs. Previously, these authors registered R. australis tupinus in dogs of a non-specified locality.
On the other hand, Trichodectes canis and Heterodoxus spiniger were collected from dogs from Central Provinces and Darien. These records provide new data regarding the distribution of these ectoparasites in Panama. The only previously reported site was Panama City. Trichodectes canis is a primary ectoparasite of Canidae, and maintains a close relationship with its host (4). This species infests dogs, coyotes, foxes and wolves in different regions from America (7), whereas in Europe (Check Republic), it has been found on Nyctereutes procyonoides (19), demonstrating its adaptability to parasites wild canids.
Unlike T. canis, H. spiniger has also been found on cats and dogs, which are alternative hosts (20, 21). These lice are primarily parasites of marsupials (e.g. kangaroos, wallabies), establishing associations with dogs only in modern times (22, 23). This species is Pantropical, nevertheless, its distribution in many Neotropicals countries is poorly documented (21).
During this study, Dermatobia hominis was the only species found to be causing myiasis in dogs. According to Bermúdez et al (24), the myiasis produced by this species in dogs corresponded to 64% of reported cases in Panama during the 2002-2005. These same authors indicated the other flies as Cochliomyia macellaria and an unidentified species of Lucilia (=Phaenicia sp.) can cause myiasis in dogs. The parasitism is commonly associated with towns near forests of other wooded sites.
In conclusion, the environmental situation in Panama, can encourage that wildlife ectoparasites parasitized dogs in absence of their native hosts. This condition may increase transmission risk of some diseases where the ticks and fleas are vectors (as ehrlichiosis and rickettsiosis).
Acknowledgements
This work was sponsored by the following projects: "Estudios de las enfermedades asociadas a ectoparásitos de Cerro Chugantí, con énfasis en Rickettsiales" (data from Western Darién and supported by SENACYT, grant 00046852), "Vigilancia Epidemiológica Panamá-Colombia" (data from East Panama and financed by Panama Government), "Proyecto Hantavirus" (data from Central Provinces Azuero, by SENACYT, MINSA, ICGES); "Determinación de los ectoparásitos de mamíferos en El Valle" (data from El Valle with partial sponsor by ICGES). Special thanks to Mauricio Caballero for sent material, Enrique Medianero for the facilities in Chiriqui highlands and Bocas del Toro; Grace Alpízar (Ministerio de Agricultura of Costa Rica) and Carlos Viquez (Instituto Nacional de la Biodiversidad, Costa Rica) for their comments; Victor Townsend (Virginia Wesleyan College) for editorial suggestions, and Marcelo Labruna (University of Sao Paulo, Brazil) for comments on an earlier version of this manuscript.
References
1. Vilá C, Savolainem P, Maldonado J, Amorin I, Rice J, et al. Multiple and ancient origins of the domestic dog. Science 1997; 276:1687-1689. [ Links ]
2. Germonpré M, Sablin M, Stevens R, Hedges R, Hofreiter M, Stiller M, Després V. Fossil dogs and wolves from Palaeolithic sites in Belguim, the Ukraine and Russia: Osteometry, ancient DNA and stable isotopes. J Arch Sci 2008; 36(2):473-490. [ Links ]
3. Leonard J, Wayne R, Wheeler J, Valadez R, Guillén S, Vilá C. Ancient DNA evidence for Old World origin of New World dogs. Science 2002; 298:1613-1616. [ Links ]
4. Guimaraes J, Tucci E, Barros-Battesti D. Brasil: Fapesp. Edit. Plêide; 2002. [ Links ]
5. Fairchild G, Kohls G, Tipton V. The ticks of Panama. In: Ectoparasites of Panama. Chicago: Field Museum of Natural History; 1966. [ Links ]
6. Onofrio V, Labruna M, Pinter A, Giacomin F, Barros-Battesti D. Comentários e chaves as espécies do gênero Amblyomma. Carrapatos de Importância Médico-Veterinária da Regiao Neotropical Brazil: Instituto Butantan, SP; 2006. [ Links ]
7. Price R, Hellenthal R, Palma R, Johnson K, Clayton D. The chewing lice world checklist and biological overview. Illinois: Natural History Survey Special Publication; 2003. [ Links ]
8. Tipton V, Méndez E. The fleas of Panama (Siphonaptera). Ectoparasites of Panama. Chicago: Field Museum of Natural History; 1966. [ Links ]
9. Guglielmone A, Estrada-Peña A, Keirans J, Robbins R. Las garrapatas (Acari: Ixodida) de la región zoogeográfica neotropical. Argentina: Inst Nac Tec Agrop; 2004. [ Links ]
10. Child J, Paddock C. Rocky Mountain Spotted Fever. Rickettsial Diseases. New York: Informa HealthCare; 2006. [ Links ]
11. Cunha N, Fonseca A, Rezende J, Rozental T, Ravacho A, Barreira J. et al. First identification of natural infection of Rickettsia rickettsii in the Rhipicephalus sanguineus tick, in the State of Rio de Janeiro. P Vet Bras 2006; 29(2):105-108. [ Links ]
12. Rovery C, Raoult D. Rickettsia conorii Infections (Mediterranean Spotted Fever, Israeli Spotted Fever, Indian Tick Typhus, Astrakhan Fever). New York: Rickettsial Diseases. Informa HealthCare; 2007. [ Links ]
13. Unver A, Perez M, Orellana N, Huang, H, Rikihisa Y. Molecular and antigenic comparasion of Ehrlichia canis from dogs, ticks and human in Venezuela. J Clin Microbiol 2001; 39:2789-2793. [ Links ]
14. Bermúdez S, Eremeeva M, Karpathy S, Samudio F, Zambrano M, Zaldívar Y et al. Detection and identification of Rickettsial Agents in ticks from domestic mammals in eastern Panama. J Med Entomol 2009; 46(4):856-861. [ Links ]
15. Labruna M, Jorge R, Sana D, Jacomo A, Kashivakura C, Furtado M. et al. Ticks (Acari: Ixodidae) on wild carnivores in Brazil. Exp Appl Acarol 2005; 36:149-163. [ Links ]
16. Labruna, M. Ecology of Rickettsia on South America. Ann N Y Acad Sci 2009; 1166:156-166. [ Links ]
17. Rodaniche E. 1953. Natural infection of the tick, Amblyomma cajennense, with Rickettsia rickettsii in Panama. Am J Trop Hyg 1953; 2(4):696-699. [ Links ]
18. Smit F. A preliminary note the occurrence of Pulex irritans and P. simulans in North America. J Parasitol 1958; 523-526. [ Links ]
19. Bádr V, Štefan P, Preisler J. Trichodectes canis (De Geer, 1778) (Phthiraptera, Ischnocera), a new ectoparasite of the raccoon dog (Nyctereutes procyonoides) in the Czech Republic. Eur J Wildlife Researches 2005; 51:133-135.
20. Colless D. Heterodoxus spiniger (Mallophaga: Boopidae) from cats in Singapore. J Parasitol 1959; 45:248. [ Links ]
21. Dantas-Torres F, Figueredo L. Heterodoxus spiniger (Enderlein, 1909) on domestic dogs (Canis familiaris, L. 1758) from the city of Recife, Pernambuco State, Brazil. Braz J Vet Res Ann Sci 2007; 44(2):77-80. [ Links ]
22. Thompson G. The distribution of Heterodoxus spiniger (Enderlein). Pap Proc Roy Soc Tasmania 1940; 27-31. [ Links ]
23. Emerson K, Price R. Evolution of Mallophaga on Mammals. In: Coevolution of Parasitic arthropods and mammals. New York: John Wiley & Son; 1985. [ Links ]
24. Bermúdez S, Espinosa J, Cielo A, Clavel F, Subía J, Barrios S, Medianero E. Incidence of myiasis in Panama during the eradication of Cochliomyia hominivorax (Coquerel 1858) (Diptera: Calliphoridae). Mem Inst O Cruz 2007; 102(6):675-679. [ Links ]