Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268On-line version ISSN 1909-0544
Rev.MVZ Cordoba vol.16 no.1 Córdoba Jan./May 2011
ORIGINAL
Effects of high temperature on production in layer chickens supplemented with vitamins C and E
Efectos de la alta temperatura sobre la producción en gallinas ponedoras suplementadas con vitaminas C y E
1Universidad Central "Martha Abreu" de Las Villas. Faculty of Agricultural Sciences. Department of Pathophysiology. Carretera a Camajuaní Km 5½. Santa Clara, Villa Clara. Cuba.
*Correspondencia: joachim@uclv.edu.cu
Recibido: Noviembre de 2009; Aceptado: Octubre de 2010.
Abstract
Objetive. To determine the effects of high temperature, the high humidity and the index on production performance in layer chickens supplemented with vitamins C and E. Materials and methods. The experiment was carried out from July 14th to August 15th. A total of 720 L33 layer chickens, 39 weeks old, were divided at random into four groups (180 birds/group), by replicates (n=4): Control Group) was fed with a basal diet and treatment groups were fed with the basal diet supplemented with either 150 mg of l-ascorbic acid/g of diet (Group Vit. C) or 150 mg of dl-α-tocopherol acetate /kg, of diet (Group Vit. E), and 150 mg of l-ascorbic acid /kg of diet plus 150 mg of dl-α-tocopherol acetate/kg of diet (Group Vit C + E). Results. Egg/bird were higher (p<0.05) in all treatment groups when compared to control group, but p value was highly significant in vitamin E treated group. Likewise, the laying index was different (p<0.05) in all treatment groups when compared to control, and P value was highly significant in vitamin E treated group. Although, viability was not affected by vitamin C, vitamin E and vitamin C+E groups when compared to control (p>0.05). However, feed consumption and conversion were different (p<0.05) in treatment groups when compared to control group. Conclusions. Dietary supplementation with 150 mg vitamin C and/or 150 mg vitamin E increased production performance in heat stressed layer chickens.
Key words: Layer chickens, animal feed, vitamin C, vitamin E, eggs production. (Sources: DeCs, AIMS, IEDCYT).
Resumen
Objetivo. Investigar los efectos de la alta temperatura y humedad relativa y su índice sobre el rendimiento productivo en las gallinas ponedoras suplementada con las vitaminas C y E. Materiales y métodos. Se realizó el experimento desde 14 de Julio a 15 de Agosto. 720 gallinas ponedoras L33, y de 39 semanas de edad, fueron dividas en cuatro grupos de 180 aves. Se suministró un grupo con dieta basal (Control) y los grupos de tratamientos fueron suministrados con dieta basal y suplementada con 150 mg de acido l-ascórbico/kg de dieta (grupo vitamina C), 150 mg de acetato dl-α-tocoferol/kg de dieta (grupo vitamina E), mientras el último grupo, se suministró 150 mg de acido l-ascórbico/kg de dieta mas 150 mg de acetato dl-α-tocoferol/kg de dieta (grupo vitamina C+E). Resultados. Huevo/ave fueron significativamente (p<0.05) mayor en todos los grupos tratados en comparación con el control, pero el p fue más alta en el grupo tratado con vitamina E. De mismo modo se observó una diferencia (p<0.05) significativa en el índice de postura en todos los grupos tratados en comparación con el control. Aunque la viabilidad no fue afectada en todos los grupos. Sin embargo, el consumo y la conversión alimentaria fueron diferentes (p<0.05) significativamente en todos los grupos tratados en comparación con el control. Conclusiones. La suplementacion dietética de 150 mg de vitamina C y/o 150 mg de vitamina E aumentó el rendimiento productivo en las gallinas ponedoras sometidas a estrés calórico.
Palabras clave: Gallinas ponedoras, alimentación animal, vitamina C, vitamina E, producción de huevos. (Fuentes: DeCs, AIMS, IEDCYT).
Introduction
In hot climates, periods of high temperatures have a negative effect on the health and performance of domestic animals. Poultry farming is no exception and the effect of stress caused by elevated temperatures can result in heavy economic losses from increased mortality and reduced productivity (1). For birds to perform at their optimum capacity they need to among other factors to be in homeostasis with their environment through the maintenance of thermobalance. Thermobalance is the equilibrium between the heat produced and the heat given out by living organism, and this is at its maximal physiological level within the thermoneutral range of any given specie. Birds, like mammals are homoeothermic, they produce heat to maintain a relatively constant body temperature and may permit certain variations within their temperature range without significant perturbation (1).
Normally, the chicken's body temperature is 41.5°C, but will fluctuate somewhat depending upon the temperature of its environment (2), while the established thermoneutral zone for birds reared in the tropical regions ranges between 18-24 Holik (3). Maintaining a constant body temperature is not a problem when air temperature is at least 10-15 degrees less than body temperature. But air movement is critical. A bird can only give off heat to its environment if the temperature of that environment is cooler than the bird. If heat produced by the birds is not moved away from them and out of the poultry house quickly, it will be more difficult for them to avoid heat stress (2).
The changes in the biomass as result of the continuous global warming has a deleterious effect on domestic animals in general, and birds in particular. Ambient temperature (AT) above 25 is stressful for birds, but more stressful is the fluctuations caused by this environmental thermal changes, especially when it is accompanied by high relative humidity (RH), as this unleash various pathophysiological response in birds (4,5). Furthermore, it has been demonstrated that this response induces heat stress in chickens, and thus lead to disturbance in production (6).
The free radicals generated as result of high AT and RH, overtax the anti-oxidant system of the animal and thus reduce their viability. The large quantity of free radicals thus liberated, initiate lipid peroxidation in cytomembranes Altan et al (7), thereby, causing damage at the cell level Ramnath et al (8). During heat stress, the environmental parameters of AT and RH in general and temperature humidity index (THI) in particular, have been reported to be an invaluable tool in the presumptive diagnosis of the animal state of health, and is also relevant in evaluating the adaptability of the animal (9,10).
Environmental fluctuations as a result of variation in AT below or above the thermal comfort zone has been reported to be a important element in the evaluation of the negative effects of stress factors that affects the organism (11). Altan et al (7) reported that high AT and RH, increases heat stress and are responsible for the increase in rectal/body temperature (RT). Although a great deal of research has been done concerning the responses of poultry to high ambient temperatures, the role that RH play on intensifying or modifying these responses has received little attention and particularly, air velocity.
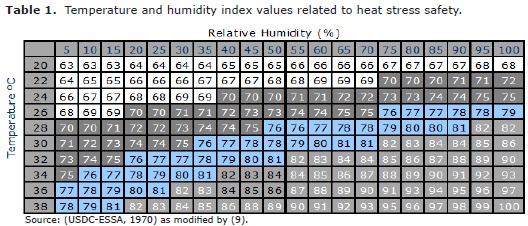
Relative humidity is rarely included as an experimental variable or even measured for information purposes. Such information is important because in poultry-producing regions, high temperatures can often be accompanied by a range of RH, which can markedly affect the degree of heat stress experienced by the bird (12). De Basilio et al (13) observed that with an AT between 38 to 40°C and RH between 50 and 55%, chickens RT could be elevated to between 45 to 48°C leading to death by heat wave or acute stress with subsequent decrease in production efficiency and economic gains. However, it was Tao and Xin (9) for the first time that adapted THI for a function in poultry using wind speed as a variable, and called this index; Temperature-Humidity-Velocity Index (THVI, Table 1). They also established several stages of thermal comfort values such as: normal ≤ 70, alert from 70 to 75, danger from 76 to 81, and emergency ≥ than 82, based on the bird's body temperature variation, based on the following mathematical model according to Tao and Xin (9):
THVI = (0.85 × DBT + 0.15 × WBT) × V-0.058
Where: THI = Temperature, Relative Humidity Index and Air Velocity;
DBT = Dry bulb temperature (°C);
WBT = Wet bulb temperature (°C).
V = Air velocity.
Thus, 70 were established as a standard threshold value for THVI in poultry.
Furthermore, De Moraes et al (14) reported that assessing the impact of specific climate on livestock performance is a difficult task. Superficial general analysis of impacts can be based on the animal response. However, combining the relationship between livestock performance and the thermal environment is needed for more precise assessment, and to provide adequate quantitative and qualitative performance evaluation. Researchers have tried to mitigate the effect of heat stress by changing the environment and diets of laying chickens. Though environmental approach through modification of housing is the best option for optimal performance, it is nevertheless highly capital intensive, thereby making nutritional strategy a viable alternative. Nutritional strategy during heat period is based on diets balancing in order to cover the needs of stressed birds for ideal amino acids (protein), energy and electrolytes (12,15). For this purpose, vitamin C and vitamin E are used in the poultry diet because of their anti-oxidant properties in the neutralization of the free radicals generated during heat stress (8).
Though poultry are renal synthesizers of vitamin C, but its quantity becomes insufficient during heat stress as a result of increased rate of usage in combating the free radicals thus generated. On the other hand vitamin E has been reported in the participation of the supply of egg precursors in plasma, while at the same time decreasing serum ACTH concentration (16). Furthermore, Sahin et al (16) observed that dietary supplementation of vitamin E increased fertility and layability in layer chickens, while it caused reduction in the process of lipid peroxidation. The aim of this study therefore, was to investigate the possible beneficial effects of dietary vitamin C and vitamin E supplementation on production performance in laying chickens subjected to acute and chronic heat stress.
Material and methods
Experimental site. The study was performed at the poultry production unit of "Las casas II", located at 5½ Km along Santa Clara and Camjauni highway in the province of Villa Clara; it is located between 22°53' N and 82°02' W, with an altitude between 90-100 meters above sea level. Total precipitation during this period was 327.2 cm, with an average air velocity of 3.15 m/s.
Experimental birds. A total of 720 and 39 weeks old commercial L33 layer chickens, were used as subjects for the experiment. The birds were divided in completely randomized design into four groups of 180 each, and each group was further divided into four replicates of 45 birds. One group was fed with basal diet (Control Group) and treatment groups were fed with the basal diet supplemented with either 150 mg of l-ascorbic acid /kg of diet (Vit. C group), 150 mg of α-dl-tocopherol acetate /kg of diet (Vit. E group), while the last group was supplemented with 150 mg of l-ascorbic acid /kg of diet plus 150 mg of α-dl-tocopherol acetate/kg of diet (Vit C + E group). Vitamin C and vitamin E used were from a commercial company (VMD, n.v./s.a, Arendonk, Belgium).
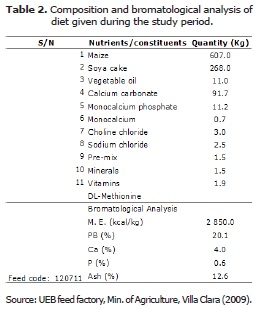
The birds received a basal diet of 110 g/bird/day, and water ad libitum. Feed constituents and bromatological analysis of the basal diet are shown in table 2. The basal diet contained 2850 kcal/kg metabolic energy (ME) and 20.1% crude protein (CP), 4.0% Ca, 0.60% P and 12.6% ash. This diet exceed slightly the nutrient requirements recommended by the standing committee on the scientific evaluation of dietary reference intakes of the National Research Council (17).
Experimental procedures. The AT and HR were measured daily using a standard thermometer (Harris, England) with a 42 calibration and a standard hygrometer (Harris, England) with a 50 calibration. All production parameters measured during this period were also carried out daily.
Statistical analyses. The PC STATISTICA 8.0 package was used and data were analyzed by One-way Anova through the general-linear-models procedure of the Statistical Analysis System. Means were compared by Duncan's post-hoc test (p<0.05) (18).
Results
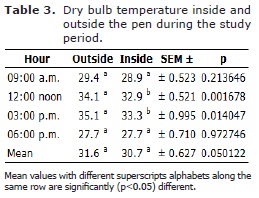
Dry bulb temperature and WBT data are shown in tables 3 and 4. There were no difference (p>0.05) in the overall average DBT inside and outside the pen, even though the dry-bulb showed a minimum temperature of 27.7°C inside and outside the pen house at 6.00 pm, the maximum temperature inside was 33.3°C and outside was 35.1°C at 3.00 pm.
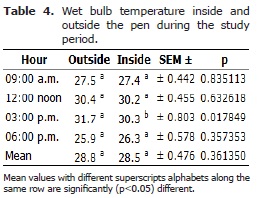
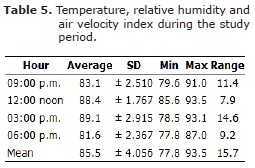
There was a significant difference DBT outside and inside the pen, between 12-3 pm (p<0.05). Even though, the WBT showed minimum values of 26.3°C and 25.9°C inside and outside the pen house, respectively. Similarly, the WBT showed minimum values of 26.3 and 25.9 inside and outside the pen house, respectively. In addition, the maximum temperature values recorded for inside and outside the pen were 30.3°C and 31.7°C, respectively at 3.00 pm (p<0.05). There was no significant difference in the overall WBT between inside and outside the pen house (p>0.05). The minimum THVI value recorded was 77.8, at 6.00 pm, and the maximum value was 93.5 at 12.00 noon (Table 5).
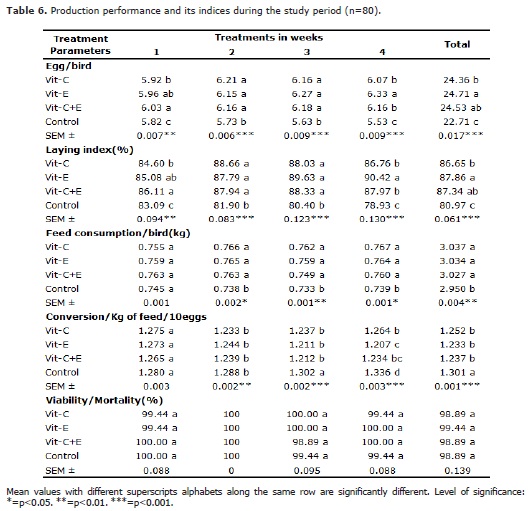
The highest range value of 14.6 during the experimental period was recorded at 3.00 pm. The average THVI value was 85.5 during the same period. Production performance parameters are presented in table 6. Egg/bird were higher in all treatment group when compared to control (p<0.05), but p value was particularly highest in vitamin E treated group. Overall egg/bird increased consistently in all groups from highly significant difference (p<0.01) in week 1 to very highly significant (p<0.001) difference from weeks 2 to 4. Likewise, the laying index was significant in all treatment groups when compared to control (p<0.05), and p value was particularly highest in vitamin E treated group. Overall laying index increased consistently in all groups from highly significant difference (p<0.01) in week 1 to very highly significant difference from weeks 2 to 4 (p<0.001). Feed consumption and conversion were significantly different in vitamin C, vitamin E and vitamin C+E supplemented groups when compared to control group (p<0.05).
Viability/mortality was not affected by vitamin C, vitamin E and vitamin C+E supplemented groups when compared to control (p>0.05).
Discussion
The dry and wet-bulb temperatures, for inside and outside the pen house were higher than the recommended thermo neutral zone of 18-24°C, that Holik (3) established for poultry in the tropical regions. Likewise, the THI in this experiment were outside the normal zone of ≤ 70 that Tao and Xin (9) established for poultry. The response of chickens at high temperatures differs with different relative humidity. It has been reported that high temperature accompanied by high humidity is more detrimental to layer performance than high temperature with low humidity. At the same time, constant high temperature of 30-32°C is more deleterious to birds than cyclic or alternating temperatures of 30-32°C by day and 25°C by night. Feed conversion in broilers is subject to marked fluctuations because of seasonal as well as ambient temperature changes.
All studies indicate that high temperatures reduce the efficiency of utilizing feed energy for productive purposes. Layers not only eat less at high temperature, but also produce less per unit of intake, especially at temperatures above 30°C. The single or combined dietary supplementation with vitamin C and vitamin E of laying chickens exposed to heat stress significantly improved production performances of feed consumption, conversion and egg/bird/day. Supplementation, of vitamin E alone into diets appeared to be more beneficial for laying hens during heat stress, probably, due to its concurrent function as fertility factor (16).
Vitamin C has been demonstrated to be a powerful antioxidant that acts through a two way mechanism, that is, through its conversion to L-dehydroascorbic acid, a particularly inert radical. This reaction is reversible and the interconversion of these molecules forms a redox system and the basic physiology if their actions, because both show vitamin C activity. The other route, is the formation of an ascorbate radical that destroys free radicals generated by oxygen, which includes the hydroxyl (OH*), mono-oxygen (O*) and the superoxides (O2*) and also in the transfer of radical equivalents from lipid phases to aqueous compartment. In realizing this function, the vitamin enters into a synergistic action with other protective enzymes such as, catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GSHPx). Puthpongsiriporn et al (19), confirmed in vitro that the addition of vitamin C, the rate of proteolytic induction by hydrogen peroxide (H2O2) and the destruction of SOD. In its scavenging function for free radicals generated in the cell membranes, the vitamin helps in the conversion of the oxidized form of vitamin E to its stable form through a non-enzymatic reaction. Similarly, vitamin E has also been demonstrated to be an antioxidant that scavenges the free radicals generated in cell membranes that participates in tissular degeneration (20).
The vitamin participates in a tripartite interaction together with selenium, an integral chemical complex of the enzyme GSHPx as protagonists, while the poly unsaturated fatty acids serve as the antagonist (GSHPx, 21). The synergic effects between these two vitamins are particularly efficient for reducing production of reactive oxygen species. Because radical reactions are exergonic, they contribute to the failure of thermoregulatory process in hyperthermia observed during heat stress. Consequently, dietary supplementation of birds with vitamin C, vitamin E or a combination of these two anti-oxidant compounds would attenuate the deleterious heat-induced-oxidative stress. Vitamin E supplementation of diets containing high amounts of polyunsaturated fatty acids may prevent feed oxidation and may contribute to egg formation. These beneficial protective effects of vitamins were evidenced by increases of egg/bird/day; feed intake and efficiency in treatment groups in comparison to control chickens.
This study is in agreement with the findings of Sahin and Kucuk (22), who reported that a combination of 200 mg of vitamin C and 250 mg of vitamin E provides the greatest performance in japanese quails reared under heat stress. That combination can be considered as a protective management practice in a poultry diet, ameliorating the detrimental effects of heat stress. In the same way, Ciftci et al (23), reported that vitamin E can alleviate the depression in egg production in heat stressed laying chickens. However, our report is not in agreement with the findings of Sosnowka-Czajka et al (24), who found that dietary supplements of vitamin C (40 mg/kg) and vitamin E (70 mg/kg) did not increase the tolerance of broilers to high ambient temperatures and failed to offset the negative effects of hyperthermia. Probably due to low doses of the vitamins supplemented or specific factors that affects the biosynthesis or turnover of both vitamins (25). Besides, Sahin et al (26) have demonstrated that a combination of 250 mg of vitamin C and 250 mg of vitamin E provides the greatest performance in japanese quails reared under heat stress. Furthermore, Ciftci et al (23) reported that the combination of vitamins C and E can attenuate the heat induced oxidative damage at the cell level. These authors concluded that the positive effects were evidenced by increases of growth performances, egg production and improvement of egg qualities in comparison to non-supplemented birds. A probable indication, that the two antioxidants potentiate their action during the inactivation process of reactive oxygen species.
Based on these results, it is concluded that dietary supplementation of laying chickens with vitamins C and E singly or in its combined form, can attenuate heat stress induced oxidative damage. This is evidenced by the increase in the productive parameters of the treatment groups when compared to control.
Acknowledgement
The authors are grateful to the management of the commercial poultry layer farm "La Casas II" for their technical assistance.
References
1. St-Pierre NR, Cobanov B, Schnitkey GX. Economic Losses from Heat Stress by US Livestock Industries. J Dairy Sci 2003; 86(E):E52-E77. [ Links ]
2. Sottnik J. Climatical factors and their effect on production in animal housing. In: ASAE Annual International Meeting/ CIGR XVth World Congress; Chicago, Illinois, USA: ASAE editors; 2002. [ Links ]
3. Holik V. Management of laying hens to minimize heat stress. Lohmann Information 2009; 44(1):16-29. [ Links ]
4. Sritharet N, Hara H, Yoshida Y, Hanzawa K, Watanabe S. Effect of heat stress on histological features on pituicytes and hepatocytes, and enzyme activities of liver and blood plasma in Japanese quail (Coturnix japonica). J Poult Sci 2002; 39:167-178. [ Links ]
5. Simon MS. Reducing heat stress problems. World Poult 2003; 19(3):16-17. [ Links ]
6. Estrada-Pareja MM, Marquez-Giron SM, Restrepo LF. Effect of temperature and relative humidity on productive parameters and heat transfer in broiler chickens. Rev Col Cienc Pec 2007; 20:288-303. [ Links ]
7. Altan O, Pabuccuaoglu A, Konyalioglu S, Bayracktar H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br Poult Sci 2003; 44(4):545-550. [ Links ]
8. Ramnath V, Rekha PS, Sujathja KS. Amelioration of heat stress induced disturbances of antioxidant defense system in chicken by Brahma Rasayana. Evid Based Complement. Alternat Med 2008; 5(Supl 1):77-84. [ Links ]
9. Tao X, Xin H. Temperature-humidity-velocity-index for market size broilers. Nevada, USA: Proceedings of the ASAE Annual International Meeting; 2003. [ Links ]
10. Karaman S, Tarhan S, Ergunes G. Analysis of Indoor Climatic Data to Assess the Heat Stress of Laying Hens. IJNES 2007; 1(2):65-68. [ Links ]
11. Ozkan O, Akbas Y, Altan O, Altan A, Aylan V, Ozkan K. The effect of short term fasting on performance traits and rectal temperature of broiler during the summer season. Br Poult Sci 2003; 44:88-95. [ Links ]
12. Balnave D. Challenges of accurately defining the nutrient requirements of heat-stressed poultry. In: World's Poultry Science Association Invited Lecture. Poult Sci 2004; 83:5-14. [ Links ]
13. De Basilio V, Vilariño M, León A, Picard M. Efecto de aclimatacion precoz sobre la termotolerancia de pollo de engorde sometido a estres calorico en condicion de clima tropical. Rev Cienc 2001; 11(1):60-68. [ Links ]
14. De Moraes SRP, Yanagi T, De Oliveira ALR, De Nazare MS. Cafe MB. Classification of the Temperature and Humidity Index (THI), aptitude of the region, and conditions of comfort for broilers and layer hens in Brazil. Iguassu Falls, Brazil: Livestock environment VIII. ASAE Eight International Symposium; 2008. [ Links ]
15. Daghir NJ. Nutritional strategies to reduce heat stress in broilers and broiler breeders. Lohmann information 2009; 44(1):6-15. [ Links ]
16. Sahin K, Sahin N, Yaralioglu S. Effects of vitamin C and vitamin E on lipid peroxidation, blood serum metabolites and mineral concentrations of laying hens reared at high ambient temperature. Biol Trace Elem Res 2002; 85:35-45. [ Links ]
17. DRI. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington DC: National Academy Press; 2005. [ Links ]
18. Rabe-Hesketh S, Everitt B. Statistical analyses using stata. 3rd ed. Washington DC: CRC Press LLC; 2004. [ Links ]
19. Puthpongsiriporn U, Scheideler SE, Sell JL, Beck MM. Effects of vitamin E and C supplementation on performance, in vitro lymphocyte proliferation and antioxidant status of laying hens during heat stress. Poult Sci 2001; 80:1190-1200. [ Links ]
20. Bollingier-Lee S, Williams PEV, Whitehead CC. Optimal dietary concentration of vitamin E for alleviating the effect of heat stress on egg production in laying hens. Br Poult Sci 1999; 40:102-107. [ Links ]
21. DRI. Dietary reference intakes for vitamin C, vitamin E, selenium and carotenoids. Washington DC: National Academy Press; 2000. [ Links ]
22. Sahin K, Kucuk O. Effects of vitamin C and vitamin E on performance, digestion of nutrients and carcass characteristics of Japanese quails reared under chronic heat stress (34°C). J Anim Physiol Anim Nutr 2001; 85:335-341. [ Links ]
23. Ciftci M, Nihat Ertas O, Guler T. Effects of vitamin E and vitamin C dietary supplementation on egg production and egg quality of laying hens exposed to a chronic heat stress. Revue Med Vet 2005; 156(2):107-111. [ Links ]
24. Sosnowka-Czajka E, Skomorucha I, Herbut E. Effect of dietary vitamin supplements on productivity and physiological parameters of broiler chickens exposed to elevated ambient temperature. Warsaw, Poland: ISAH; 2005. [ Links ]
25. Maurice DV, Lightsey SF, Abudabos A, Toler JE. Factors affecting ascorbic acid biosynthesis in chickens: III. Effect of dietary fluoride on L-gulonolactone Oxidase activity and tissue ascorbic acid (AsA) concentration. J Anim Physiol Anim Nutr 2002; 86:383-388. [ Links ]
26. Sahin K, Sahin N, Onderci M, Gursu MF, Issi M. Vitamin C and E can alleviate negative effects of heat stress in Japanese quails. Food Agric Environ 2003; 1(Supl 2):244-249. [ Links ]