Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268On-line version ISSN 1909-0544
Rev.MVZ Cordoba vol.16 no.2 Córdoba May/Aug. 2011
ORIGINAL
Effect of age and coasting period on oocytes quality and their in vitro development from prepubertal cattle
Efecto de la edad y la hora de la aspiración sobre la calidad oocitaria y el desarrollo in vitro en hembras bovinas prepúberes
Sandra Bernal U,1 MVZ. Ángela Gonella D,1* MVZ. Diego Valbuena,2 MV. Rubén Mendoza,2 MV. Juan Molina,3 M.Sc. Liliana Chacón J,4 Ph.D.
1Universidad de Ciencias Aplicadas y Ambientales UDCA, Facultad Ciencias Agrarias. Bogotá, Colombia.
2Central de procesamiento de semen y núcleo de mejoramiento Genético Las Camelias. Puerto Araujo, Colombia.
3Intervet International B.V.
4Universidad de La Salle, Facultad de Medicina Veterinaria. Bogotá, Colombia.
*Corresponding: angelamvz@gmail.com
Recibido: Junio de 2010; Aceptado: Febrero de 2011
Abstract
Objective. This study evaluated the effect of age and coasting period over oocyte quality and their posterior development under in vitro conditions from prepubertal Bos indicus crossbred donors. Material and methods. Donors females received a norgestomet implant and estradiol benzoate. Four days later a unique dosage of 150 IU of eCG was administered. Three coasting periods (24, 48, and 56 h) and four ages (3, 6, 10, and 12 months) were proved. All antral follicles were aspirated and an in vitro culture proceed was done. Results. 439 follicles were aspirated, of which 385 (87.7%) were 2-5 mm, 41 (9.33%) were 5-10 mm, and 13 (2.3%) were >10 mm. After aspiration, a total of 373 oocytes (84.9%) were recovered, finding differences (p<0.05) between averages of 6 (9.3) and 10 months old animals (32.3). 285 (76.4 %) recovered oocytes were subjected to in vitro process. Cleavage values were significantly higher (p<0.05) in 10 (27.1%) and 12 months animals (26.8%). Although the number of transferable embryos was low, there were differences between ages (p<0.05) obtaining a higher percentage in age 3 (12.6%). Conclusions. A coasting period higher than 24 h has a negative effect on oocyte quality. Some oocytes from 3 months old calves were competent for in vitro embryo development; however, higher numbers of embryos were produced from 10 and 12 months of age prepuber females, indicating they have higher competency in vitro.
Key words: ECG, in vitro embryo production, oocytes donation, stimulation. (Sources: DeCS, AIMS).
Resumen
Objetivo. Este estudio evaluó el efecto de la edad y el período de aspiración folicular sobre la calidad del oocito y su posterior desarrollo in vitro con donadoras prepúberes Bos indicus mestizas. Materiales y métodos. Las hembras donantes recibieron un implante de norgestomet y benzoato de estradiol y cuatro días más tarde una dosis única de 150 UI de eCG. Las aspiración folicular se realizó en tres tiempos (24, 48, y h 56) y en cuatro edades (3, 6,10, y 12 meses). Todos los folículos antrales fueron aspirados y se realizaron procedimientos de maduración, fertilización y cultivo in vitro. Resultados. Se aspiraron 439 folículos en total, de los cuales 385 (87.7%) fueron de 2-5 mm, 41 (9.33%) fueron de 5-10 mm, y fueron 13 (2.3%) > 10 mm. Se recuperó un total de 373 oocitos (84.9%) y se encontraron diferencias significativas (p<0.05) entre los promedios de los animales de 6 (9.3) y 10 meses (32.3). 285 (76.4%) oocitos fueron sometidos a los procesos in vitro. La tasa de división fue significativamente mayor (p<0.05) en los animales de 10 (27.1%) y 12 meses (26.8%). Aunque el número de embriones transferibles fue bajo, existieron diferencias entre las edades (p<0.05), obteniendo el porcentaje más alto los animales de 10 meses (12.6%). Conclusiones. Un período de aspiración superior a 24 h tiene un efecto negativo sobre la calidad del oocito. Algunos oocitos procedentes de terneras de 3 meses fueron competentes en el desarrollo in vitro, sin embargo, un mayor número de embriones se produjeron con hembras de 10 y 12 meses de edad.
Palabras clave: Donación de óvulo, eCG, estimulación, producción de embriones in vitro. (Fuentes: DeCS, AIMS).
Introduction
The potential of commercial application of in vitro embryo production (IVP) programs in fetal (1) and prepubertal females (2) offers accelerated genetic gain through a reduction in the generation interval since these animals represent a rich source of germ plasm (3-5). Furthermore, the use of IVP in prepubertal females is mainly useful in the genetic improvement of cattle breeds where puberty is achieved later, such as Bos indicus breeds (6). Bos indicus breeds and their crossbreeding with Bos taurus offers broad characteristics of tolerance and resistance to the extreme conditions in the tropics (7). These selective breeding effects (heterosis) on in vitro embryo production have been evaluated by Camargo et al (8) who evidenced that blastocyst development of Gyr (Bos indicus) and crossbred embryos is greater than with Holstein embryos.
Through IVP, it would be possible to produce embryos from crossbred prepubertal donors with greater genetic merit for dairy or beef crossbred herds (6). Nevertheless, previous studies have reported that developmental competence of oocytes from prepubertal donors is low in both Bos taurus and in Bos indicus (2, 6, 9-11). Low follicular activity needs to be stimulated in some donors, mostly by using Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH), their combinations or equine chorionic gonadotropin (eCG) (12-14). Some studies have demonstrated that the administration of gonadotropin increases the number of viable oocytes and the follicular development per session. These have been administrated at different schemes, dosages, and products with different results (10, 15-20). Few studies have been conducted with prepubertal Bos indicus and Bos indicus crossbreeds as donors of oocytes. But none have evaluated the effect of the time interval between hormonal stimulation and oocyte recovery (coasting period) (21,22) and the donor age in prepubertal Bos indicus crossbred females. A correct costing period is required to have an excellent process of in vivo prematuration and in vivo final maturation (22). This study was developed to evaluate the effect of age of the donor at the OPUâs time and the coasting period after gonadotropin stimulation over oocyte quality and their posterior development under in vitro conditions from prepubertal Bos indicus crossbred donors.
Materials and methods
Gonadotropin stimulation. Twenty one Bos indicus crossbred (F1, Gyr x Holstein) prepubertal females, received a norgestomet sub cutaneous implant (Day 0; Crestar; Intervet International B.V.) and estradiol benzoate (1 mg; Syntex Laboratories). Four days later (day 4) a sole total dosage of 150 IU of Equine Chorionic Gonadotrophin (eCG; Folligon; Intervet International B.V.) was administered to each female. After the follicular aspiration, the implant was removed.
Chemical reagents. Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich Chemical Co.
Oocyte Recovery. After stimulation, three coasting periods were established. The first group of animals was aspirated 24 hours after the eCG administration, the second group of animals was aspirated 48 hours after the gonadotropin administration, and the last group was aspirated 56 hours after the eCG administration. Prior to follicular aspiration, the animals were anesthetized with a xilacine and ketamine combination. The ovaries were exposed by midventral laparotomy, and all antral follicles were aspirated using a 16-gauge needle attached to a 10 mL disposable syringe (15).
The oocytes were aspirated in a medium consisted of 0.9% saline sterile solution supplemented with 1% penicillin and streptomycin, 10% fetal bovine serum (FBS), and 5 IU/mL of sodium heparin. Immediately the content of the syringe was transferred into an Em-Con filter and washed 2-3 times. Cumulus-oocyte complexes (COCs) were collected, evaluated, and washed in this medium and then subjected to in vitro maturation. The oocytes were classified under the following parameters: Quality I (QI) - oocytes with homogeneous cytoplasm and at least four compact cumulus cell layers; Quality II (QII) - oocytes with some granulation in cytoplasm and less than four compact cumulus cell layers; Quality III (QIII) - oocytes with homogeneous or some granulation in cytoplasm and some cumulus cells expanded; Quality IV (QIV) - oocytes with expanded cumulus; Quality V (QV) - naked oocytes.
in vitro maturation (IVM). Maturation was performed from 22 to 26 hours. The maturation medium was bicarbonate-buffered TCM-199, containing 10% FBS, 0.01 mL/IU of FSH, (0.1 IU/mL of LH, 0.055 mg/mL of pyruvate, 100 IU/mL of penicillin, and 100 µg/ mL of streptomycin) (23). Oocytes from individual donors were placed into cryotubes containing 700 µL of maturation medium covered with 300 µL of mineral oil. Each cryotube was gassed with a mixture of 5% CO2 and 5% O2 before being sealed. They were stored and transported at 38°C during approximately ten hours to the in vitro fertilization (IVF) laboratory. At the laboratory, each cryotube cap was unscrewed and the cryotubes were transferred to the incubator in a humidified atmosphere of 5% CO2 in air at 38.5°C.
in vitro fertilization (IVF). After maturation, matured COCs were transferred into 50 µL droplets under a mineral oil fertilization medium (TALP-FERT, 23) enriched with BSA, piruvate, and antibiotics. Motile spermatozoa were obtained by frozen-thawed spermatozoa on a Percoll discontinuous density gradient. Spermatozoa were diluted to obtain a final concentration of 1 x 106 cells/mL and coincubated with COCs for 18 h in a humidified atmosphere of 5% CO2 in air at 38.5°C.
in vitro embryo culture (IVC). Presumptive zygotes were denuded by agitation for a minute and cultured under mineral oil in 50 µL droplets of CR2aa medium (24) enriched with 10% fetal calf serum, and 1 mg/mL of BSA. Every 48 h, half of the medium was replaced. Seven days post-insemination embryo development was evaluated.
Statistical analysis. This study involved four age groups of animals: 3, 6, 10, and 12 months. Each group was conformed by three groups of different aspiration hours after stimulation 24, 48, 56 h. All groups were managed as a single experiment. Statistical computations were performed by using variance and student-t test analysis on all data (SAS 9.1.3 Version, SAS Institute Inc.)
Results
The prepubertal status of the animals was confirmed by the absence of the corpus luteum or corpus albicans prior to the time of follicle aspiration (6). Before aspirations, the follicles were counted, measured, and classified according to their size: < 5 mm, 5-10 mm, and > 10 mm (25). From the total number of 439 aspirated follicles, 385 (87.7%) were 2-5 mm, 41 (9.33%) were 5-10 mm, and 13 (2.3%) were >10 mm. There were no differences among the coasting periods (p<0.05), but differences were found (p<0.05) between 6 and 10 months animals for the aspirated follicle number: 10.5 (8.7%) and 37.3 (34.67%), respectively. Differences were not found (p>0.05) for the 5-10 and > 10 mm sizes. After aspiration, a total of 373 oocytes (84.9%) were recovered, and differences were located (p<0.05) between the oocite recovery averages of 6 (9.3) and 10 months (32.3) (Table 1).
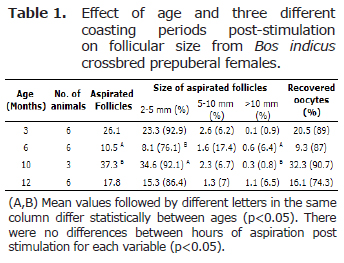
The oocytes were classified according to their quality. Although the number of oocytes for the QI and QII were low, significantly different values (p<0.05) (Tables 2 and 3) were found among coasting periods; 24 h being the period with the highest averages. Furthermore, there were no differences among ages for these qualities.
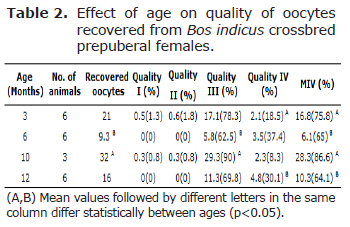
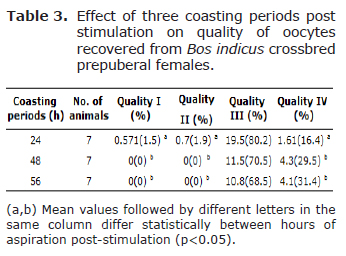
Quality III had the greatest number of oocytes and differences were found (p<0.05) (Tables 2 and 3) only between the ages 6 (62.5%) and 10 months (90%). For the QIV, there were differences between the ages 3 and 12 months with the latter having the highest value (30.15%). Moreover, 56 h was the coasting period with the highest averages for this quality (4.1,31.4%). From the total recovered oocytes, 285 (76.4%) were subjected to in vitro maturation/in vitro fertilization/in vitro embryo culture (IVM/IVF/IVC).
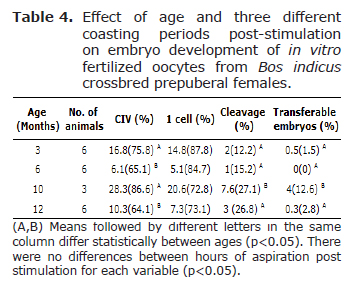
The cleavage values were significantly higher (p<0.05) in 10 (27.1%) and 12 months of ages animals (26.8%). Although the number of transferable embryos was low, there were differences between ages (p<0.05) and, 10 months of age animals obtained the highest percentage (12.6%). There were no differences (p<0.05) among coasting periods for cleavage and transferable embryo values (Table 4).
Discussion
This is the first study to investigate the effect of time interval between hormonal stimulation and oocyte recovery (coasting period, 21) and the donor age in prepubertal Bos indicus crossbred cattle.
When the ovarian response was evaluated, we found that follicular development was similar among the different coasting periods. In contrast, Blondin et al (21) evidenced that a longer coasting period resulted in a greater percentage of 5-10 mm follicles in stimulated cows. We only found differences for the total aspirated follicles and follicles of 2-5mm between 6 and 10 months of age, of which 10 months had greater values (92.1% for <5mm follicles). These results differ from Snel-Oliveira et al (26), who did not find differences in the total follicle number (≥ 3mm) among Nelore donors at 10, 11, and 12 months, or differences in stimulation treatments. We did not find differences with the 12 months of age animals in which the number of follicles was lower than 10 months of age ones (26).
Molina et al (27) showed that hormonal stimulation with gonadotropins increased oocyte quality and their in vitro development. On the contrary, Snel-Oliveira et al (26) reported that there were no differences in the total recovered oocytes or in the viable oocytes between ages and stimulation treatments. In the present study, qualities I and II of recovered oocytes were only found in the 24-h coasting period, the quantity of expanded follicles increased with the coasting period after stimulation, most oocytes were quality III, and 10 months of age animals had the highest quantity of QIII oocytes. Thus, indicating that oocyte quality declines with the increase of the coasting period. In contrast, Blondin et al (21) found no effect of 33 h and 48 h coasting periods on types of COCs from stimulated cows. Oropeza et al (28), reported 53, 61 and 60% of competent oocytes from un-stimulated Bos taurus (Holstein) calves at 6-7, 9-10, and 11-12 months of age, as well as blastocyst rates of 1, 9, and 10% for the same ages; therefore, showing that age affects cleavage rates.
In this study, the cleavage and transferable embryos were higher in older donors (9.5-10 and 11.5-12 months) and these results agree with previous reports. For example, the oocytes collected from non-stimulated calves at 1-4 months (9), between 5 and 9 months of age (16), and from 6-8 months B. taurus (11), lacked embryonic competence to produce viable transferable embryos. Additionally, Oropeza et al (28) evidenced that oocytes from Bos taurus calves between 6-7 months of age produced lower blastocyst development rate. Furthermore, Camargo et al (6), showed that non-stimulated B. indicus crossbred 4-7 month old calves are less competent than oocytes derived from cows, but oocytes from non-stimulated 9 to 14 month old heifers achieve similar developmentally competent as oocytes from cows. Camargo et al (6) showed that oocytes from non-stimulated B. taurus (Holstein) females reached competence at 11 months of age, suggesting that these oocytes have similar competence to those of cows. The reduced developmental competence of oocytes from prepubertal calves is attributed to a deficient expression of facilitative glucose transporters, insufficient protein translation (28), size, ultrastructure, metabolism, and cytoplasmic maturation (29).
Through this study, we observed broad individual variability among donors of the same age. We found values among 0 and 64 recovered oocytes and embryo production rates from 0 to 7 in the same groups. These observations have been previously reported in prepubertal animals by other authors (15, 26, 30, 31). In conclusion, the results suggest that a coasting period higher than 24 h has a negative effect on oocyte quality and on their subsequent in vitro development. Additionally, some oocytes from 3 month old calves were competent for in vitro embryo development. However, a higher number of embryos were produced by 10 and 12 month prepuberal females, thus, indicating higher in vitro competence.
Acknowledgments
This work was supported by the Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnología COLCIENCIAS-BID. No. 1254-07-12211, contrato No. 269-2002.
References
1. Chohan KR, Hunter AG. in vitro maturation, fertilization and early cleavage rates of bovine fetal oocytes. Theriogenology 2004; 61:373-380. [ Links ]
2. Kelly JM, Kleemann DO, Walker SK. A comparision of the in vitrodevelopment of cow and calf oocites. Theriogenology 2003; 59:449(Abstract). [ Links ]
3. Galli C, Duchi R, Crotti G, Turini P, Ponderato N, Colleoni S, Lagutina I, Lazzari G. Bovine embryo technologies. Theriogenology 2003; 59(2):599-616. [ Links ]
4. Mapletoft RJ, Hasler JF. Assisted reproductive technologies in cattle: a review. Rev Sci Tech Off Int Epiz 2005; 24(1):393-403. [ Links ]
5. Morton KM. Developmental capabilities of embryos produced in vitro from prepubertal lamb oocytes. Reprod Domest Anim 2008; 43(Suppl 2):137-43. [ Links ]
6. Camargo LSA, Viana JHM, Sá WF, Ferreira AM, Vale Filho VR. Developmental competence of ooctytes from prepubertal Bos indicus crossbred cattle. Anim Reprod Sci 2005; 85: 53-59. [ Links ]
7. Fisher AE, Bernal DP, Gutierrez-Robayo C, Rutledge JJ. Estimates of heterosis for in vitro embryo production using reciprocal crosses in cattle. Theriogenology 2000; 54:1433-1442. [ Links ]
8. Camargo LSA, Viana JHM, Sá WF, Ferreira AM, Ramos AA, Freitas C, Vale Filho VR. Developmental competence of oocytes obtained from Bos taurus and Bos indicus dairy cows raised in tropical climate. Reprod Fert Dev 2006; 18:243-244. [ Links ]
9. Kauffold J, Amer HAH, Bergfeld U, Weber W; Sobiraj A. The in vitro Developmental Competence of Oocytes from Juvenile Calves is Related to Follicular Diameter. J Reprod Dev 2005; 51(3):325-332. [ Links ]
10. Ptak G, Matsukawa K, Palmieri C, Della Salda L, Scapolo PA, Loi P. Developmental and functional evidence of nuclear immaturity in prepubertal oocytes. Hum Reprod 2006; 21(9):2228-2237. [ Links ]
11. Majerus V, Lequarré AS, Ferguson EM, Kaidi S, Massip A, Dessy F, Donnay I. Characterization of embryos derived from calf oocytes: kinetics of cleavage, cell allocation to inner cell mass, and trophoectoderm and lipid metabolism. Mol Reprod Dev 2000; 57:346-352. [ Links ]
12. Bols PEJ, Leroy JLMR, Viana JHM. Technical and biological aspects of ultrasound-guided transvaginal oocyte retrieval in the cow: an overview. Acta Sci Vet 2005; 33(supl1): 103-118. [ Links ]
13. Petyim S, Bage R, Hallap T, Bergqvist AS, Rodriguez-Martinez H, Larsson B. Two different Schemes of twaice wakly ovum pick-up in dairy heifers: effect on oocite recovery and ovarian function. Theriogenology 2003; 60: 175-188. [ Links ]
14. Merton JS, de Roos APW, Mullaart E, de Ruigh L, Kaal L, Vos PLAM, Dieleman SJ. Factors affecting oocite quality and quantity in comercial application of embryo technologyes in cattle breeding industry. Theriogenology 2003; 59: 651-674. [ Links ]
15. Taneja M, Bols PEJ, Van de Velde A, Ju JC, Shreiber D, Tripp MW, Levin H, Echelard Y, Yan X. Developmental competence of juvenile calf oocytes in vitro and in vivo: influence of donor animal variation and repeated gonadotropin stimulation. Biol Reprod 2000; 62:206-213. [ Links ]
16. Presicce GA, Senatore EM, De Santis G, Stecco R, Terzano GM, Borghese A, De Mauro GJ. Hormonal stimulation and oocyte maturational competence in prepuberal Mediterranean Italian buffaloes (Bubalus bubalis). Theriogenology 2002; 57 (7):1877-1884. [ Links ]
17. Ptak G, Tischner M, Bernabo N, Loi P. Donor-Dependent Developmental Competence of Oocytes from Lambs Subjected to Repeated Hormonal Stimulation. Biol Reprod 2003; 69(1):278-285. [ Links ]
18. Techakumphu M, Promdireg A, Na-Chiengmai A, Phutikanit N. Repeated oocyte pick up in prepubertal swamp buffalo (Bubalus bubalis) calves after FSH superstimulation. Theriogenology 2004; 61(9):1705-11. [ Links ]
19. Chaubal SA, Molina JA, Ohlrichs CL, Ferre LB, Faber DC, Bols PEJ, Riesen JW, Tian X, Yang X. Comparison of different transvaginal ovum pick-up protocols to optimise oocyte retrieval and embryo production over a 10-week period in cows. Theriogenology 2006; 65: 1631-1648. [ Links ]
20. Goodhand KL, Staines ME, Hutchinson JSM, Broadbent PJ. In vivo oocyte recovery and in vitro embryo production from bovine oocyte donors treated with progestagen, oestradiol and FSH. Anim Reprod Sci 2000; 63: 145-158. [ Links ]
21. Blondin P, Bousquet D, Twuagiramungu H, Barnes F, Sirard MA. Manipulation of follicular development to produce developmentally competent Bovine oocytes. Biol Reprod 2002; 66:38-43. [ Links ]
22. Dieleman SJ, Hendriksen PJM, Viuf D, Thomsen PD, Hyttel P, Knijn HM, Wrenzyck C, Kruip TAM, Niemann H, Gadella BM, Bevers MM, Vos PLAM. Effects of in vivo prematuration and in vivo final maturation on developmental capacity and quality of pre-implantation embryos. Theriogenology 2002; 57: 5-20. [ Links ]
23. Choi YH, Carnevale EM, Seidel GE Jr, Squire EL. Effects of gonadotropins on bovine oocytes matured in TCM-199. Theriogenology 2001; 56(4):661-70. [ Links ]
24. Camargo LS, Freitas C, de Sa WF, de Moraes Ferreira A, Serapiao RV, Viana JH. Gestation length, birth weight and offspring gender ratio of in vitro-produced Gyr (Bos indicus) cattle embryos. Anim Reprod Sci 2010; 120(1-4):10-5. [ Links ]
25. Seneda MM, Esper CR, Andrade ER, Binelli M, Max MC, Oliveira JA, Garcia JM. Relationship between follicle size after FSH treatment and efficiency of oocytes recovery. Anim Reprod 2005; 2:78-182. [ Links ]
26. Snel-Oliveira MN, Costa PD, Malagoni JD, Rumpf R. Estimulação Hormonal, Punção Folicular Transvaginal e Avaliação Ovocitária em bezerras Pré-Púberes da raça Nelore (Bos taurus indicus). R Bras Zootec 2003; 32: 106-114. [ Links ]
27. Molina JR, Benoit AM, Lkhagvadorj S, Anderson LL. Hypothalamic differentiation in prepuberal beef heifers: Effects of gonadotropin-releasing hormone and estradiol benzoate on luteinizing hormone secretion. Livest Sci 2009; 120(1):13-24. [ Links ]
28. Oropeza A, Wrenzycki C, Herrmann D, Hadeler KG, Niemann H. Improvement of the Developmental Capacity of Oocytes from Prepubertal Cattle by Intraovarian Insulin-Like Growth Factor-I Application. Biol Reprod 2004; 70(6):1634-1643. [ Links ]
29. Salamone DF, Damiani P, Fissore RA, Robl JM, Duby RT. Biochemical and developmental evidence the ooplasmic maturation of prepubertal bovine oocytes is compromise. Biol Reprod 2001; 64:1761-1768. [ Links ]
30. Mapletoft RJ, Steward KB. Adams GP. Recent advances in the superovulation in cattle. Reprod Nutr Dev 2002; 42(6):601-611. [ Links ]
31. Peterson AJ, Lee RS. Improving successful pregnancies after embryo transfer. Theriogenology 2003; 59(2):687-97. [ Links ]














