Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268
Rev.MVZ Cordoba vol.17 no.3 Córdoba Sept./Dec. 2012
ORIGINAL
Lipofundin 20% induces hepatic lipid peroxidation in New Zealand white rabbits
Lipofundin 20% induce peroxidación lipídica hepática en conejos Nueva Zelanda blancos
Livan Delgado R,1* M.Sc, Ángela Fraga P,1 M.Sc, María Bécquer V,1 M.Sc, Ana Vázquez L,2 Ph.D.
1Center of Studies for Research and Biological Evaluations, Pharmacy and Food Sciences College, University of Havana, Havana 13600, Cuba.
2Center of Molecular Immunology, Havana, Cuba.
*Correspondencia: ldelgado@ifal.uh.cu
Recibido: Septiembre de 2011; Aceptado: Febrero de 2012.
ABSTRACT
Objective. The aim of the present work was to evaluate the effects of Lipofundin 20% on lipid peroxidation markers in the liver of New Zealand white rabbits. Materials and methods. The animals were treated with an intravenous injection (2 ml/kg) of the lipid emulsion during 8 days through the marginal ear vein. At the end of the experiment some lipid peroxidation parameters and lipid profile were tested through spectrophotography. Results. Lipofundin was found to induce a significant (p<0.05) increase of malondialdehyde, total hydroperoxides, and peroxidation potential. Also, high levels of total cholesterol, triglycerides, LDL - cholesterol and HDL-cholesterol were observed in treated animals compared with the control group (p<0.05). Conclusions. Data proved that Lipofundin induces hepatic lipid peroxidation in rabbits, mainly through a mechanism which involves an induction of hyperlipidemia.
Keywords: Hyperlipidemia, Lipofundin, lipid peroxidation, oxidative stress, rabbits (Source: DeCS).
RESUMEN
Objetivo. El objetivo del presente trabajo fue evaluar los efectos del lipofundin 20% sobre marcadores hepáticos de peroxidación lipídica en conejos blancos Nueva Zelanda. Materiales y métodos. Los animales fueron tratados con una inyección intravenosa (2 ml/kg) de la emulsión lipídica durante 8 días por la vena marginal de la oreja. Al final del experimento algunos marcadores de peroxidación lipídica y el perfil lipídico fueron espectrofotométricamente determinados. Resultados. Se observó que el lipofundin indujo un incremento significativo (p<0.05) de malonildialdehído, hidroperóxidos totales y el potencial de peroxidación. También, altos niveles de colesterol total, triglicéridos, colesterol de LDL y colesterol de HDL fueron observados en los animales tratados respecto a los del grupo control (p<0.05). Conclusiones. Los resultados demostraron que el Lipofundin 20% induce peroxidación lipídica hepática en conejos, principalmente a través de un mecanismo que involucra la inducción de hiperlipidemia.
Palabras clave: Conejos, estrés oxidativo, hiperlipidemia, lipofundin, peroxidación lipídica (Fuente: DeCS).
INTRODUCTION
Lipid peroxidation (LPO) was first studied in the 1930’s in relation to food deterioration, but since then, there has been increasing evidence showing the involvement of free radicals in biology, leading to renewed attention on LPO with a wider scope in the fields of chemistry, biochemistry, nutrition and medicine (1,2), amongst others. Further studies revealed that, like proteins, carbohydrates, and nucleic acids, lipids are targets of reactive oxygen species (ROS) and become oxidized to render cytotoxic products (3). Several oxidized products have been studied and also used as LPO biomarkers, such as malondialdehyde (MDA) and lipoperoxides (LOOH) (4).
Artificial fat emulsions are widely used in parenteral nutrition. The soya oil-based fat emulsions represent a major part of energy and are also a necessary source of essential fatty acids in the mentioned therapy (5,6). Lipofundin 10% constitutes a frequently indicated fat emulsion as a source of calories for patients requiring parenteral nutrition, but preclinical investigations demonstrated that Lipofundin 20% induces atherosclerotic lesion formation in rabbits (7). Our group also demonstrated that this fat emulsion induces a systemic LPO in rabbits (8), but the effects on hepatic LPO have not been assessed. Therefore, the purpose of the present work is to evaluate the effects of Lipofundin 20% on lipid profile and hepatic biomarkers of LPO in New Zealand White rabbits (NZB).
MATERIALS AND METHODS
Animals. Standard NZW male rabbits, weighing 2.0-2.5 kg and 12 weeks old, were obtained from CENPALAB (Mayabeque, Cuba). Rabbits were housed under conventional conditions exposed to a 12 hr light-dark cycle with free access to water and food. Animal studies were performed with approval of the Pharmacy and Food Sciences College Institutional Animal Ethical Committee. All procedures were in accordance with the European Union Guidelines for animal experimentation.
Lipofundin composition. Lipofundin MCT/LCT 20% (Braun Melsungen AG, Melsungen, Germany) is a lipid emulsion containing soya oil 100 g, medium-chain triglycerides 100 g, glycerol 25 g, egg lecithin 12 g, α-tocopherol 170 ± 40 mg, and sodium oleate/water for injection in sufficient quantity to 1000 ml.
Experimental design. Two groups of 10 rabbits were used in the study. The first group received an intravenous injection of phosphate-buffered saline solution (PBS), pH 7,4 (control group), and the second one received a slow intravenous injection of 2 ml/kg of Lipofundin MCT/LCT 20%, as an infusion during 1-2 min (7). This procedure was repeated daily during a period of 8 days. On day 9, the animals were anesthetized with ketamine hydrochloride (5 mg/kg i.m.), and euthanized with an overdose of sodium pentobarbital (90 mg/kg, i.v.). (Abbott Laboratories, México SA de CV, México). Then, the liver was perfused with NaCl 0.9% solution at 4°C.
Liver homogenate preparation. The hepatic right lobe of each animal was extracted and homogenized in 20mM KCl/histidine buffer, pH 7.4, 1:10 w/v using a tissue homogenizer (Edmund Bühler LBMA, Germany) at 4°C and centrifuged for 10 min at 12000 g. Supernatants were taken for biochemical determination.
Serum sample collection. Blood samples (1 ml) were obtained on day 0 and 9 (at the end of the study), for biochemical analyses. Blood was withdrawn from the rabbit’s marginal ear vein. These samples were immediately centrifuged at 2500 g, at 4°C for 10 min. The serum was collected and aliquots were stored at -80°C until analysis.
Serum lipid assay. Total cholesterol, triglycerides, LDL-cholesterol and HDL-cholesterol Serums were determined using commercial enzymatic kits (Randox, Crumlin, UK).
Redox biomarkers determination. All biochemical parameters were determined through spectrophotometric methods using a Pharmacia 1000 Spectrophotometer (Pharmacia LKB, Uppsala, Sweden). Total protein levels were determined using the method described by Bradford (9) with bovine albumin serum as standard.
Total hydroperoxides (ROOH) were measured through Bioxytech H2O2-560 kit (Oxis International Inc., Portland, OR, USA). The assay is based on the oxidation of Fe2+ to Fe3+ by hydroperoxides under acidic conditions. Ferric ions bind with indicator xylenol orange (3.3'-bis(N,N-di(carboxymethyl)-aminomethyl)-o-cresolsulfone-phtalein, sodium salt) to form a stable colored compound, which can be measured at 560 nm.
MDA Concentration was determined using the LPO-586 kit obtained from Calbiochem (La Jolla, CA, USA). In the evaluation, the production of a stable chromophore after 40 min of incubation at 45°C was measured at 586 nm. For control, freshly prepared solutions of malondialdehyde bis [dimethyl acetal] (Sigma St Louis, MO, USA) were employed and evaluated under identical conditions (10).
In order to determine susceptibility to lipid peroxidation and total reactive antioxidant power (TRAP), the samples were incubated with a solution of copper sulphate (final concentration 2 mM) at 37°C for 24 h. The peroxidation potential (PP) was calculated by subtracting the MDA levels before the induction of LPO from the one obtained at 24h (11).
Statistical analysis. Statistical analysis was performed using the SPSS program for Windows (version 11.5, SPSS Inc). Bartlett’s Box-test was used to test the homogeneity of variance. Differences between groups were determined by independent student’s t-test (two-tailed). Data was expressed as the mean ± standard deviation (SD). A P-value <0.05 was considered as statistically significant.
RESULTS
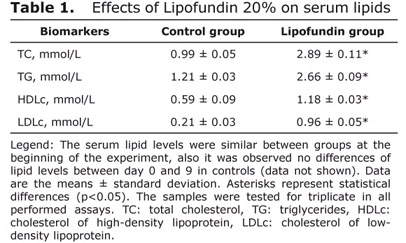
Serum total cholesterol, triglycerides, LDL-cholesterol and HDL-cholesterol levels showed a significant increase (p<0.05) in those animals who were treated during 8 days with the lipid-rich emulsion Lipofundin, compared with the control (Table 1). These parameters were determined on day 0 and statistical differences were not shown (data not shown).
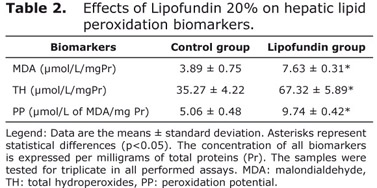
Table 2 shows the behavior of hepatic biomarkers of LPO in both groups. The damages on lipids were significantly (p<0.05) modified after 8 days of Lipofundin administration compared to the non-treated group. At the end of the experimental period the MDA levels, one of the end-products of LPO, were higher in lipofundin treated animals compared with controls. Total ROOH levels were also significantly higher (p<0.05) in the animals that were administered with lipofundin than in non-treated specimens. Besides, lipofundin treatment also caused an increase in PP compared with the control group (p<0.05).
DISCUSSION
After lipofundin administration, high levels of triglycerides, total cholesterol, LDL-cholesterol and HDL-cholesterol serums were observed. Indeed, there is a causal relationship between elevated plasma lipids and the occurrence of LPO (12).
Lipofundin 20%-induced hyperlipidemia could be associated with the high content of triglycerides in this emulsion. High levels of exogenous triglycerides promote ApoB100 and cholesterol synthesis, and eventually the assembly of very low-density lipoproteins (VLDL) (13). In fact, these results are in accordance with our previous report (8), while it is known that lipofundin 10% caused a 60% increase in total serum cholesterol after parenteral administration in a human study (5).
In addition, there is a mutual lipid and apolipoprotein exchange between serum lipoproteins and infused triglyceride/phospholipid particles (14). The increase of HDL-cholesterol may be determined by a physiological response against the elevated LDL-cholesterol levels.
This study demonstrated that lipofundin-induced hyperlipidemia induces liver oxidative stress. Strong evidence of the involvement of increased free radical production in the onset of hyperlipidemia has been reported previously (15). Also, lipofundin was recently demonstrated to induce an increase of serum lipids in rabbits (7) and in rats (16).
In vitro LPO mechanisms, dynamics, and products have been studied extensively and are now fairly well understood and documented (17). Lipid hydroperoxides and hydrogen peroxide (H2O2) generated by LPO play a central role in many diseases such as atherosclerosis, mainly during endothelial dysfunction (18). Transition metals (iron or copper) may produce H2O2 decomposition and cause the generation of the highly toxic and reactive hydroxyl radical (•OH), which reacts with cellular components (19). High ROOH concentration detected in rabbits treated with lipofundin 20% may be a blank for free transition metals attack. ROOH decomposition affects the delicate balance between antioxidants and pro-oxidant factors, which can leads to oxidative stress states.
An increasing MDA level in those animals treated with lipofundin 20% was also detected in this study. Our data is in accordance with the criteria that this end-product of LPO and is strongly associated with the development of hyperlipidemia (20).
Lipofundin-induced high serum lipid levels, especially atherogenic ones such as cholesterol and LDL allow to explain, in part, the fact that these were higher in animals administered with LPO products than in controls. MDA is considered as a major epitope of oxidized LDL (21), suggesting that lipofundin 20% induces an increase in LPO closely associated with an elevation of LDL particles and related ApoB100-containing lipoproteins. Also, PP increase in livers from treated rabbits reinforces the criteria that LPO is determinant in the loss of redox hepatic status in former animals which were under Lipofundin 20% treatment.
In conclusion, the present study demonstrated that lipofundin 20% induces hyperlipidemia, thereby promoting hepatic LPO. Our data shows novel evidences of lipofundin-induced oxidative damages on hepatic lipids. These results reinforce the attractive characteristics of lipofundin to be used as an experimental inductor of LPO in rabbits.
REFERENCES
1. Niki E. Free radicals in the 1900's: from in vitro to in vivo. Free Radic Res 2000; 33:693-704. [ Links ]
2. Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med 2009; 47:469-484. [ Links ]
3. Dalle-Donne I, Rossi R, Colombo R, Gustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem 2006; 52: 601-623. [ Links ]
4. Abuja PM, Albertini R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin Chim Acta 2001; 306: 1-17. [ Links ]
5. Hailer S, Wolfram G. Influence of artificial fat emulsions on the composition of serum lipoproteins in humans. Am J Clin Nutr 1986; 43:225-233. [ Links ]
6. Calder PC, Jensen GL, Koletsko BV, Singer P, Wanten GJA. Lipid emulsions in parenteral nutrition of intensive care patients: current thinking and future directions. Intensive Care Med 2010; 36:735-749. [ Links ]
7. Delgado L, Acosta E, Fraga A, Bécquer MA, Soto Y, Cama V et al. Lipofundin-induce hyperlipidemia promotes oxidative stress and atherosclerotic lesions in New Zealand White rabbits. Int J Vasc Med 2011; 2012:1-7. [ Links ]
8. Delgado L, Acosta E, Hernández-Matos Y, Bécquer MA, Vázquez AM, Fernández-Sánchez E. High levels of lipid peroxidation induced by Lipofundin administration correlates with atherosclerotic lesions in rabbits. Pharmacol online 2010; 3:727-736. [ Links ]
9. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248-254. [ Links ]
10. Erdelmeier I, Gerard-Monnier D, Yadan JC, Chaudiere J. Reactions of N-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Mechanistic aspects of the colorimetric assay of lipid peroxidation. Chem Res Toxicol 1998; 11:1184-1194. [ Links ]
11. Ozdemirler G, Mehmetcik G, Oztezcan S, Toker G, Sivas A, Uysal M. Peroxidation potential and antioxidant activity of serum in patients with diabetes mellitus and myocardial infarction. Horm Metab Res 1995; 27:194-196. [ Links ]
12. Jain KS, Kathiravan MK, Somani RS, Shishoo CJ. The biology and chemistry of hyperlipidemia. Bioorg Med Chem 2007; 15:4674-4699. [ Links ]
13. Carlson LA. Studies on the fat emulsion Intralipid®. I. Association of serum protein to Intralipid® triglycerides particle. Scand J Clin Lab Invest 1980; 40:139-144. [ Links ]
14. Horwich TB, Hernández AF, Dai D, Yancy CW, Fonarow GC. Cholesterol levels and in-hospital mortality in patients with acute decompensate heart failure. Am Heart J 2008; 156:1170-1176. [ Links ]
15. Jones DP. Redefining oxidative stress. Antioxid Redox Signal 2006; 8:1865-1879. [ Links ]
16. Richard D, Kefi K, Barbe U, Bausero P, Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol Res 2008; 57:451-455. [ Links ]
17. Delgado L, Fraga A, Bécquer MA, Hernández-Matos Y. Lipofundin induces hyperlipidemia and oxidative stress in male Sprague Dawley rats. Vet World 2012; 5:133-137. [ Links ]
18. Leonarduzzi G, Chiarpotto E, Biasi F, Poli G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol Nutr Food Res 2005; 49:1044-1049. [ Links ]
19. Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG et al. The lipid whisker model of the structure of oxidized membranes. J Biol Chem 2008; 283:2385-2396. [ Links ]
20. Tani S, Nagao K, Anazawa T, Kawamata H, Furuya S, Fuji T et al. Association of plasma level of malondialdehyde-modified low-density lipoprotein with coronary spastic angina: implication of acute coronary events. Int J Cardiol 2009; 135:202-206. [ Links ]
21. Tavori H, Aviram M, Khatib S, Musa R, Nitecki S, Hoffman A et al. Human carotid atherosclerotic plaque increases oxidative state of macrophages and low density lipoproteins, whereas paraoxonase (PON1) decreases such atherogenic effects. Free Radic Biol Med 2009; 46:607-615. [ Links ]