Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268
Rev.MVZ Cordoba vol.18 no.2 Córdoba May/Aug. 2013
ORIGINAL
Microbiota of Vibrio sp. in the hepatopancreas of cultured white pacific shrimp (Litopenaeus vannamei)
Vibrio sp., microbiota en el hepatopáncreas del camarón blanco del Pacífico (Litopenaeus vannamei)
Renata Albuquerque C,1,2* Ph.D, Giselle Cristina S,2 M.Sc, Rayza Lima A,1 B.Sc, Edirsana Maria RC,2 M.Sc, Regine Helena SFV,1,2 Ph.D.
1Federal University of Ceará, Fish Engineering Department. Fortaleza-Ceará, Brazil.
2Federal University of Ceará, Sea Sciences Institute. Fortaleza-Ceará, Brazil.
*Correspondencia: renata.albuq@gmail.com
Recibido: Marzo de 2012; Aceptado: Noviembre de 2012.
ABSTRACT
Objective. The present study aimed to investigate the presence of vibrios in the hepatopancreas of cultured shrimp. Materials and methods. Vibrios from the hepatopancreas of fifteen samples of five specimens each, of apparently healthy Pacific white shrimp (Litopenaeus vannamei) were isolated, identified and quantified. Results. The vibrio density ranged from 430 to 2,400 MPN g-1 (rs MPN cm-1=-0.114; rs MPN g-1 = 0.211). Thirty isolations were obtained, most of which belonged to the species V. cholerae (n=11) and V. parahaemolyticus (n=7). Conclusions. The outcomes of the present study suggest that, even in the absence of symptoms of vibriosis, the microbiota of the hepatopancreas of cultured shrimp may include sucrose positive and negative vibrios.
Key words: Hepatopancreas, Vibrio, white shrimp (Source:OED).
RESUMEN
Objetivo. El presente estudio tuvo como objetivo investigar la presencia de vibrios en el hepatopáncreas del camarón de cultivo. Material y métodos. En este estudio, fueron aislados, identificados y cuantificados los vibrios del hepatopáncreas de 75 camarones blancos del Pacífico (Litopenaeus vannamei), aparentemente sanos, oriundos de un cultivo en la región nordeste de Brasil. Quince muestras, cada una consta de cinco camarones, se pusieron a prueba. Resultados. La densidad de Vibrio varió de 430 a 2.400 NMP g-1 (rs NMP cm-1 = -0.114; rs NMP g-1 = 0.211). Treinta aislamientos fueron obtenidos, la mayoría de los cuales pertenecían a la especie V. cholerae (n=11) y V. parahaemolyticus (n=7). Conclusiones. Los hallazgos del presente estudio sugieren que, incluso en ausencia de síntomas de la vibriosis, la microbiota de lo hepatopáncreas del camarón de cultivo puede incluir vibrios sacarosa positivos y negativos.
Palabras clave: Camarón blanco, hepatopáncreas, Vibrio (Fuente:OED).
INTRODUCTION
Vibrios are ubiquitous in marine environments (1), and are part of the natural microbiota of aquatic invertebrates. They may colonize the exoskeleton, gills and intestines of penaeids (2), the hemolymph of crustaceans (3) and the hepatopancreas of shrimp (4).
Vibrio colonization of the digestive tract of aquatic organisms can be beneficial to the host. Sawabe et al (5) suggests that the populations of V. halioticoli present in the intestines of different species of abalone contribute to nourish the host by fermenting algal polysaccharides, converting alginic acid into acetic acid.
However, because vibrio are part of the indigenous microbiota of both marine invertebrates and their environment, and because of the opportunistic character of infections caused by vibrios (6) they represent a permanent potential source of infection for livestock.
According to Sung et al (7), the establishment of vibriosis in cultured shrimp is associated with increased levels of pathogenic vibrios in the environment, though not necessarily for the total vibrio population. V. harveyi, one of the species most frequently implicated in vibriosis, is known to cause severe infections in penaeid livestock (8).
Vibriosis in shrimp farms has also been shown to be related to the vibrio density in the animals' hepatopancreas. In an analysis of the hepatopancreas and hemolymph of infected shrimp, Soto Rodriguez et al (9) estimated the vibrio density by inducing vibriosis, but vibrio levels have not yet been established for healthy shrimp. Thus, the objective of the present study was to evaluate the vibrio sp., microbiota of apparently healthy shrimp farmed in Ceará (Northeastern Brazil) by isolating, identifying and quantifying (MPN) vibrios found in the hepatopancreas.
MATERIAL AND METHODS
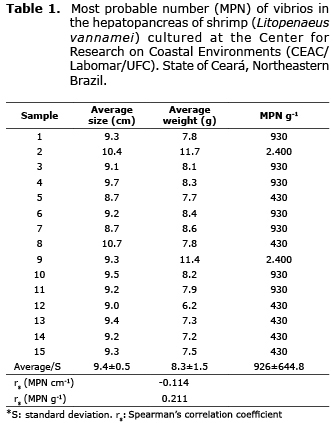
Sampling. The study included 75 adult shrimp (Litopenaeus vannamei) cultured at the Center for Research on Coastal Environments (CEAC/Labomar/UFC) in the state of Ceará, Northeastern Brazil. Sampling was done in May 2009, which corresponds to the rainy season. The water quality conditions measured were: temperature (29°C), pH (6.58) and salinity (15%). The shrimp farmed in tanks were collected with nets and transported live in 30-L containers with culture water. The time from collection to bacteriological analysis did not exceed two hours.Â
Sample preparation. Thermal shock was used as euthanasia technique by immersion in a mixture of water and ice (6:1) during 10 minutes. The shrimp were washed with 70% (v/v) alcohol and weighed. Then the carapace was opened and the hepatopancreas (HP) was removed with sterilized tweezers. Each sample consisted of HP from five specimens (total: 15 samples) diluted in 0.85% (w/v) saline in a proportion of 1:9 (w/v). Serial decimal dilutions were prepared from 10-1 to 10-4.
Quantification and isolation of vibrios. Vibrios were quantified with the MPN method (MNP.g-1), following the recommendations of Kaysner and DePaola Jr (10). Presumptive tests (PT) were performed with 1 ml of each dilution inoculated in alkaline peptone water (pH 8.5) and incubated at 35°C for 24 hours. For the confirmatory test, aliquots from positive PT tubes were inoculated, spread-plated in duplicates, in thiosulfate citrate bile sucrose (TCBS) agar and incubated at 35°C for 18 hours. The MPN of vibrios was calculated from the critical series resulting from the combination of PT-positive tubes and sucrose-positive and/or negative colonies in TCBS. The corresponding value was read from the table cited by Blodgett (11), multiplied by the average dilution and divided by 100. Results were expressed in MPN g-1. For the purpose of isolation, two sucrose-positive or negative colonies were selected from each sample and seeded in trypticase soy agar (TSA) containing 1% (w/v) NaCl.
Identification of vibrios. Pure colonies isolated in TSA (1% w/v NaCl) were submitted to biochemical identification (12) and morphotype characterization by Gram staining.
Statistical analysis. The relation between variation in MPN g-1 and average sample weight and size was tested with Spearman's correlation coefficient (rs).
RESULTS
Vibrio density ranged from 430-2,400 MPN g-1 (Table 1), and the higher loads were those determined in the samples with the larger specimens (average weight: 11.7 and 11.4 g). The rs MPN cm-1 was -0.114 and rs MPN g-1 was 0.211 (Table 1).
Out of the five species identified, the most frequent was V. cholerae (n=11; 36.7%), followed by V. parahaemolyticus (n =7; 23.3%), V. neptunius (n= 5; 16.7%), V. xuii (n=4; 3.3%) and V. coralliilyticus (n= 3; 10.0%).
DISCUSSION
There was no significant correlation between morphometric parameters (size and weight shrimp) and quantification of Vibrio (rs MPN cm-1=-0.114; rs MPN g-1=0.211) In the present study. The relationship between weight and microbiota of Vibrio in shrimp hepatopancreas has been reported by Soto Rodríguez et al (9). In Shrimp Diseases, the authors cited found that vibrio density in hepatopancreas individuals weighing 8-12 g had a greater bacterial loads than individuals weighing 0.26-4.0 g.
The observation of vibrios in the HP of healthy shrimp (Table 1) confirms the findings of Gomez-Gil et al (4) who studied the natural microbiota in penaeid shrimp with a mean weight of 10.66 g, and concluded that a large variety of vibrios in the HP of healthy shrimps are not necessarily an indication of disease. The authors added that the tissues of L. vannamei display a diversified microbiota, including saccharose-fermenting bacteria in the intestinal tract.
Leãnos et al (13) found similar total vibrio loads in healthy and infected shrimp during a complete 60-day culture cycle. However, sick shrimp presented an increase in luminescent vibrios suggesting that infection involves the multiplication of a specific population of pathogens. Considering that the HP of healthy shrimp may be colonized by vibrios, the authors proposed a safety level of 104 CFU/HP for the prevention of disease outbreaks during the first 30 days of culture.
The bacterial load in shrimp HP is often quantified by standard plate count (7,13). In contrast, the method used in this study (MPN) does not provide a direct bacterial count, but determines the density of viable microorganisms in the sample and is therefore indicated for samples with an expected bacterial concentration of less than 10 UFC g-1 (14). Thus, the mean vibrio density (9.26x102 MPN g-1) observed in the present study is not comparable to the indexes (1.30 x 104 and 105 CFU g-1) reported by Gomez-Gil et al (4) for vibrios in healthy L. vannamei. The lack of comparability makes it difficult to say whether our MPN g-1 values are high or low.
The presence of V. cholerae in the HP of apparently healthy penaeids matches results published by Suzita et al (15). According to the latter, V. cholerae occurs in salt and brackish water, freely or associated with zooplankton and algae, and may adhere strongly to the digestive tract of marine organisms.
In contrast with these findings, Sung et al (16) failed to isolate V. parahaemolyticus from the HP of asymptomatic shrimp. In any case, colonization by V. parahaemolyticus of the HP of apparently healthy shrimp represents a potential hazard.
Even if V. cholerae and V. parahaemolyticus (represented in this study by 60% of the isolates) were parts of the indigenous microbiota of shrimp HP, both species have been implicated in vibriosis outbreaks on shrimp farms (17). Aside from risk to shrimp culture, the presence of V. choleare and V. parahaemolyticus in seafood have been associated with food borne diseases (18).
On the other hand, vibrio colonization of the HP is not the only possible source of vibriosis in cultured shrimp. Vibrios can penetrate the epithelium and shrimp tissues may be colonized in several different ways, such as via contaminated food or carapace injury (19).
The other vibrios identified in this study, such as V. neptunius and V. xuii, were first described by Thompson et al (20) based on isolates from aquaculture environment (bivalves, fish, rotifers and shrimp). Likewise, the species V. coralliilyticus was described only recently by Ben-Haim et al (21) based on strains isolated from infected coral (Pocillopora damicornis) and larvae of Crassostrea gigas and Nodipecten nodosus. Since some of the strains used to describe V. neptunius and V. xuii have also been isolated from marine organisms occurring or farmed in Northeastern Brazil, the presence of these vibrio species in the HP of L. vannamei cultured in Ceará should come as no surprise.
The findings of the present study suggest that, even in the absence of symptoms of vibriosis, the microbiota of the hepatopancreas of cultured shrimp may include sucrose-positive and negative vibrios.
REFERENCES
1. Radjasa OK, Urakawa H, Kita-Tsukamoto K, Ohwada K. Characterization of psychrotrophic bacteria in the surface and deep-sea waters from the Northwestern Pacific Ocean based on 16S ribosomal DNA analysis. Mar Biotechnol 2001; 3:454-462. [ Links ]
2. Kannapiran E, Ravindran J, Chandrasekar R, Kalaiarasi A. Studies on luminous, Vibrio harveyi associated with shrimp culture system rearing Penaeus monodon. J Environ Biol 2009; 30:791-795. [ Links ]
3. Sizemore RK, Colwell RR, Tubiash HS, Lovelace TE. Bacterial flora of the hemolymph of the blue crab Callinectes sapidus: numerical taxonomy. Appl Microbiol 1975; 29:393-399. [ Links ]
4. Gomez-Gil B, Tron-Mayén L, Roque A, Turnbull J F, Inglis V, Guerra-Flores AL. Species of Vibrio isolated from hepatopancreas, haemolymph and digestive tract of a population of healthy juvenile Penaeus vannamei. Aquaculture 1998; 163:1-9. [ Links ]
5. Sawabe T, Setoguchi N, Inoue S, Tanaka R, Ootsubo M, Yoshimizu M et al. Acetic acid production of Vibrio halioticoli from alginate: a possible role for establishment of abalone - V. halioticoli association. Aquaculture 2003; 219:671-679. [ Links ]
6. Goarant C, Merien F, Berthe F, Mermoud I, Perolat P. Arbitrarily primed PCR to type Vibrio spp. pathogenic for shrimp. Appl Environ Microbiol 1999; 65:1145-1151. [ Links ]
7. Sung HH, Hsu SF, Chen CK, Ting YY, Chao W-L. Relationships between disease outbreak in cultured tiger shrimp (Penaeus monodon) and the composition of Vibrio communities in pond water and shrimp hepatopancreas during cultivation. Aquaculture 2001; 192:101-110. [ Links ]
8. Austin B, Zhang XH. Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett Appl Microbiol 2006; 43:119-124. [ Links ]
9. Soto Rodriguez SA, Gomez-Gil B, Lozano R, Roque A. Density of vibrios in hemolymph and hepatopancreas of diseased pacific white shrimp, Litopenaeus vannamei, from Northwestern Mexico. J World Aquac Soc 2006; 41:76-83. [ Links ]
10. Kaysner CA, DePaola Jr A. Vibrio. In: Food and Drug Administration - FDA. Bacteriological Analytical Manual. 2004. [Accessed on: 10 Julio 2010]. URL Available at: http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/UCM070830#authors. [ Links ]
11. Blodgett R. Appendix 2: most probable number from serial dilutions. In: Food and Drug Administration - FDA. Bacteriological Analytical Manual. 2006. [Accessed on: 10 Julio 2010]. URL Available at: http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ucm109656.htm#authors. [ Links ]
12. Noguerola I, Blanch AR. Identification of Vibrio spp. with a set of dichotomous keys. J Appl Microbiol 2008; 105:175-185. [ Links ]
13. Leaño EM, Lavilla-Pitogo CR, Paner MG. Bacterial flora in the hepatopancreas of pond-reared Penaeus monodon juveniles with luminous vibriosis. Aquacult 1998; 164:367-374. [ Links ]
14. Swanson KMJ, Petran RL, Hanlin JH. Culture methods for enumeration of microorganisms. In: Compendium of methods for the microbiological examination of foods. Washington DC: American Public Health Association. 2001. [ Links ]
15. Suzita R, Abdulamir AS, Bakar FA, Son R. A mini review: cholerae outbreak via shellfish. Am J Infect Dis 2009; 5:40-47. [ Links ]
16. Sung HH, Li HC, Tsai FM, Ting MM, Chao W-L. Changes in the composition of Vibrio communities in pond water during tiger shrimp (Penaeus monodon) cultivation and in the hepatopancreas of healthy and diseased shrimp. J Exp Mar Biol Ecol 1999; 236:261-271. [ Links ]
17. Haldar S, Chatterjee S, Asakura M, Vijayakumaran M, Yamasaki S. Isolation of Vibrio parahaemolyticus and Vibrio cholerae (non-O1 and 139) from moribund shrimp (Penaeus monodon) and experimental challenge study against post larvae and juveniles. Ann Microbiol 2007; 57:55-60. [ Links ]
18. Morris Jr JG. Cholera and other types of vibriosis: a story of human pandemics and oysters on the half shell. Clin Infect Dis 2003; 37:272-280. [ Links ]
19. Martin GG, Rubin N, Swanson E. Vibrio parahaemolyticus and V. harveyi cause detachment of the epithelium from the midgut trunk of the penaeid shrimp Sicyonia ingentis. Dis Aquat Org 2004; 60:21-29. [ Links ]
20. Thompson FL, Li Y, Gomez-Gil B, Thompson CC, Hoste B, Vandemeulebroecke K, Rupp GS et al. Vibrio neptunius sp. nov., Vibrio brasiliensis sp. nov. and Vibrio xuii sp. nov., isolated from the marine aquaculture environment (bivalves, fish, rotifers and shrimps). Int J Syst Evol Microbiol 2003; 53:245-252. [ Links ]
21. Ben-Haim Y, Thompson FL, Thompson CC, Cnockaert MC, Hoste B, Swings J et al. Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int J Syst Evol Microbiol 2003; 53:309-315. [ Links ]