Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268
Rev.MVZ Cordoba vol.19 no.1 Córdoba Jan./Apr. 2014
ORIGINAL
Cryptosporidium spp., comparative diagnosis and geospatial distribution in diarrheic calves from dairy farms, Valdivia, Chile
Diagnóstico comparativo y distribución geoespacial de Cryptosporidium spp., en terneros diarreicos en lecherías de Valdivia, Chile
Pamela Muñoz A,1* M.Sc, Rubén Mercado P,2 Ph.D, Gabriela Morales T,1 MV, Viviana Bravo O,1 MV, Eduardo Raffo C,1,3 MV.
1Austral University of Chile, Faculty of Veterinary Science, Valdivia, Chile.
2University of Chile, Medicine School, Santiago, Chile.
3Austral University of Chile, National Commission for Scientific and Technology Research of Chile (CONICYT) Doctorate Program in Veterinary Science, Valdivia, Chile
*Correspondence: pamela.munoz@uach.cl
Received: April 2013; Accepted: September 2013.
ABSTRACT
Objective. To determine the Cryptosporidium spp. infection frequency by using Ziehl-Neelsen and Auramine stains on samples obtained from diarrheic calves from milking farms of the Valdivia province. To compare both diagnostic tests and to determine the geospatial distribution of the infections caused by this protozoan. Materials and methods. 221 fecal samples of diarrheic calves of 24 milking farms of the Valdivia province were studied. The processing and analysis of the samples was done by Ziehl-Neelsen (ZN) and Auramine (AU) staining techniques, and the results were compared by McNemar statistical test and the concordance level was determined by kappa index. A map was also generated to determine the geospatial distribution of Cryptosporidium infections. Results. 57.9% of all the animals tested were classified as positive with the ZN stain test, while 55.6% of all the animals turned out positive for the AU stain test. The McNemar test showed no significant difference between both diagnostic techniques (p>0.05), while the kappa index showed proper concordance between tests (k=0.73). 100% of the farms studied showed protozoan presence demonstrating the broad distribution of the parasite, however, and considering the previous factor, it was not possible to determine geospatial associations for the parasite distribution. Conclusions. The infection frequency of Cryptosporidium is higher than 50% in the milking farms studied from the Valdivia province. No difference between the Ziehl-Neelsen and Auramine staining techniques was demonstrated showing very consistent results. It was possible to detect that the number of farms infected correspond to 100% of the farms analyzed.
Key words: Bovine, diarrhea, feces, infection, parasite, protozoa (Source: DeCS).
RESUMEN
Objetivo. Determinar la frecuencia de infección por Cryptosporidium spp., mediante las tinciones de Ziehl-Neelsen y Auramina en terneros diarreicos de predios lecheros de la provincia de Valdivia. Comparar ambas pruebas diagnósticas y determinar la distribución geoespacial de las infecciones causadas por este protozoo. Materiales y métodos. Se estudiaron 221 muestras fecales de terneros diarreicos pertenecientes a 24 predios de la provincia de Valdivia. El procesamiento y análisis de las muestras se realizó mediante tinción de Ziehl-Neelsen (ZN) y Auramina (AU); y ambas técnicas se compararon mediante la prueba estadística de McNemar y su nivel de concordancia se determinó mediante índice kappa. Se generó además un mapa para determinar la distribución geoespacial de las infecciones por Cryptosporidium. Resultados. Del total de animales muestreados, 57.9% resultaron positivos a ZN, mientras que 55.6% fueron positivo para AU. En la prueba de McNemar no hubo diferencia significativa entre los métodos diagnósticos estudiados (p>0.05), en tanto el índice kappa determinó una concordancia buena entre ambas pruebas (k=0.73). Del total de predios georeferenciados el 100% resultó positivo a la presencia del protozoo; demostrándose que ésta parasitosis tiene una amplia distribución; sin embargo, dado este factor, no fue posible determinar asociaciones geoespaciales sobre la distribución de éste. Conclusiones. La frecuencia de infección por Cryptosporidium supera el 50% en los predios lecheros de la provincia de Valdivia. No hubo diferencia entre las técnicas Ziehl-Neelsen y Auramina con resultados concordantes. Fue posible detectar que el número de predios infectados corresponde al 100% de los predios analizados.
Palabras clave: Bovino, diarrea, heces, infección, parásito, protozoa (Fuente: DeCS).
INTRODUCTION
Cryptosporidiosis is a parasite infection with cosmopolitan distribution, caused by the Cryptosporidium spp. protozoan, the main clinical sign of which in the host is non-hemorrhagic diarrhea (1,2). Cryptosporidiosis is considered to be a zoonotic disease, hence its importance from the public health point of view (3). In cattle, it mainly affects newborn calves that acquire the parasite orally (1.4), being the feces of newborns or young individuals with diarrhea and adult animals that act as asymptomatic carriers the main source of infection (2). Cow's teats or substitute milk buckets, which may be contaminated with the oocysts of the parasite, are another important source of infection for newborn calves (4).
With respect to the prevalence of the disease, the first reports in Chile of the discovery of Cryptosporidium in cattle, are produced in the year 1986, a study where the presence of this protozoan was determined in 12 calves from a total of 51 diarrheic animals. Then in 1989, a study conducted in the Metropolitan region found lower prevalence levels, 2.2% in healthy animals and 23% in diarrheic animals (5,6). Then in 1997, a study conducted on diarrheic calves, reported a prevalence of 30.6% in the south of Chile (6). The latest study in the country is that conducted by Diaz-Lee et al (5) in the Metropolitan region, where 205 samples of diarrheic valves belonging to 2 dairy farms were analyzed, determining an infection rate of 57%.
With regard to the laboratory diagnosis of this parasitism, in livestock animals there are different methods for detecting oocysts of Cryptosporidium, such as the modified Ziehl-Neelsen stain or the Auramine stain (5). Both stains are applied on previously concentrated feces and then are observed in an optical and ultraviolet light microscope for fluorescence, respectively (7). The Auramine technique shows advantages to the Ziehl-Neelsen stain as it is faster and more efficient method for the diagnosis of oocysts of Cryptosporidium spp., since the time investment during the staining process and the reading of the smear proves to be less, so it is mostly used for the analysis of a large number of samples or for epidemiological or epizootiological studies (8).
The objective of this study was to determine the frequency of infection by Cryptosporidium spp. in diarrheic calves from dairy farms through Ziehl-Neelsen and Auramine stains; comparing the performance of both diagnostic tests using the McNemar statistical test and the consistency of these through the kappa index; and generate a geospatial distribution map of infections caused by this protozoan in dairy calves from the Valdivia province.
MATERIALS AND METHODS
Samples. Feces samples from diarrheic calves were collected, with ages raging between 1 and 35 days, between the months of May 2010 to April 2011. These were obtained directly from the rectum of each animal and each sample was placed in 50 mL tubes containing 25 mL of ethanol at 70%, with their respective identification (number of each animal, gender, age and farm). The samples were transferred to the parasitology laboratory of the Faculty of Veterinary Science of the Austral University of Chile for further analysis.
Processing. From each sample two fecal smears were placed on a slide, one for Ziehl-Neelsen stain and another for Auramine stain, which were left to dry at room temperature to then be stained according to the protocol of Fredes et al (9) for Ziehl-Neelsen and according to Fayer and Xiao (7) for Auramine.
Geospatial distribution. All the geographical latitude and longitude coordinates where fecal samples from calves were obtained were recorded through the use of a portable GPS for civilian use. Data were obtained and registered with the WGS-84 grid system identifying coordinates and the corresponding geographical zone. The information obtained was transferred to a blank map on the Arc-Gis software generating a layer with the sampling points. Layers of information were added to this blank map such as land ownership, elevation curves, hydrology, soil and vegetation types, in order to determine risk criteria for the presence of the parasite (10).
Statistical analysis. The results were analyzed using the McNemar statistical test for correlated or dependent proportions. While the kappa (k) index was used for determining the level of concordance between the two tests (11). All the information collected was entered into a database generated in the computer program Microsoft® Excel 2003.
RESULTS
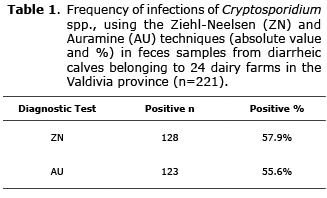
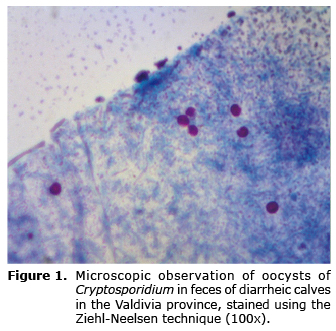
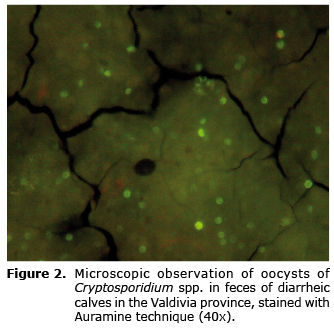
Twenty-four dairy farms from the Valdivia province were sampled. From these 221 feces samples from diarrheic calves were obtained. One hundred twenty-eight turned out positive to the Ziehl-Neelsen stain, which corresponds to 57.9% of the total samples (Table 1). The presence of abundant spherical elements with a size between 4-6 µm was seen in all positive samples, with borders delimited and stained in fuchsia (Figure 1). With respect to the Auramine stain, 123 samples were positive which corresponds to 55.6% of the total samples (Table 1). The presence of spherical elements with an approximate size between 4-6 µm was found, unevenly stained in fluorescent green (Figure 2).
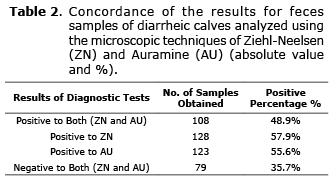
From the total of diarrheic calves studied, 48.9% turned out positive to both diagnostic tests and 35.7% were negative for both Ziehl-Neelsen and Auramine (Table 2).
Using McNemar it was determined that there was no significant difference between the two laboratory tests employed (p>0.05), insofar as the kappa index showed proper consistency between the two tests (k=0.73). Finally, from the total of farms analyzed (n=24) 100% was positive to the presence of the protozoan.
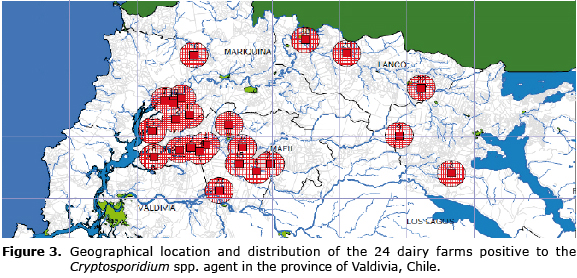
The layered map generated for the determination of geospatial risk factors did not allow conducting a differentiation since 100% of the farms recorded showed positive results for the presence of the parasite under study, so the geospatial distribution could not be associated with the specific characteristics of the geographical area (Figure 3).
DISCUSSION
The high infection level by Cryptosporidium spp., observed in dairy calves of the Valdivia province was higher when compared to the first records observed in this part of the country (5,6); where a 30.6% criptosporidosis prevalence was reported in the regions of Maule, Araucania and Los Lagos. In this study approximately twice the infection rates for the same animal species and the same age range were determined. On the other hand, in recent studies conducted in the Metropolitan Region an infection level of 49.8% was determined using the Ziehl-Neelsen technique and 56.1% using Auramine, from a total of 205 diarrheic calves belonging to two dairy farms (5). It is important to highlight the fact that in this study as well as in that conducted by Diaz-Lee et al (5), results exceeding 50% of animals positive to this protozoan agent were obtained. This proves that cryptosporidiosis is a parasitic infection that remains prevalent and will probably remerge in the country.
Regarding the McNemar test, it demonstrated that there are no significant differences between the two diagnostic techniques (p>0.05). De Quadros et al (8), in 331 feces samples from domestic animals where both techniques were compared, determined that 7.5% of the samples analyzed turned out positive for ZN and 5.7% for AU and ZN, showing that there are no significant differences between both methods. The authors also stated that the AU staining has a greater affinity for the wall of the oocysts of Cryptosporidium spp. Than the fuchsine used for the ZN stain, demonstrating that the first stain has advantages to the second as it is a more effective diagnosis method, of faster execution and reading, so it is mostly used in a large number of samples or for epidemiological studies. In contrast, the study conducted by Diaz-Lee et al (5) demonstrates that there is a difference between both methods, and the AU staining has a greater sensitivity than ZN, detecting a significant difference (p<0.05). It has been suggested that ZN method would stain a small proportion of the oocysts present in a sample and could stain a smaller number of oocysts than the AU test (11); however, this same author considered an acceptable sensitivity for both diagnostic techniques. With respect to the kappa index, it was obtained as a result that the level of concordance between the two diagnostic tests is good (k=0.73), which is consistent with the level of concordance achieved by Brook et al (12), where the k value was equal to 0.79.
Regarding the advantages and disadvantages of the techniques used, it is worth mentioning that the main advantage of ZN as a diagnostic methodology is its low cost, coupled to the fact that once the smears have been stained, there may be stored permanently, as opposed to the AU stain (12). While the disadvantage of ZN is the longer time that must be used in conventional microscopy (11,12). The quick execution and reading of the AU stain is noteworthy, but the stained smears cannot be kept permanently and a dark field microscopy equipped with ultra violet light is required for the observation of stained samples, which is not always available in clinical laboratories; and finally the fluorescence on the bottom of sample could represent a problem in the reading of smears when there is a large amount of fecal detritus present in the same (7).
On the other hand, it is important to consider that oocysts of the protozoan agent were detected in a diarrheic calf of 1 day of age followed by the death of the animal. This find is related to that recorded in the study of Diaz-Lee et al (5) who also emphasizes the fact of having investigated 2 animals positive for this protozoan on the day of their birth, i.e. before the incubation period (3 to 4 days) for this infection in cattle (2). A possible cause of finding oocysts in calves of 1 day of age has been referred to by Romero et al (4) involving the teats of the mother as a major source of infection by being contaminated with oocyst of Cryptosporidium spp., which would enter the digestive system of the calf when drinking the colostrum. The elimination of oocysts in a smaller period of time than that described in the lifecycle of this protozoan may be explained by the role of the transit host, which would be fulfilled by the calf of one day old being eliminated directly through feces. In turn, it is possible that there are other routes for transmission so it is necessary to do further research on the subject.
In relation to the geospatial distribution of infections caused by this protozoan, it was determined that the presence of oocysts of Cryptosporidium spp. was investigated in the 24 farms sampled, which demonstrates a high infection level in the Valdivia province. It was not possible to determine the risk criteria associated with the presence or absence of protozoa; since there were no groups, farms or sectors in the province negative to the agent. Similarly, the characteristics of the vegetation, soil quality and proximity to watercourses were practically shared by all farms, since all correspond to dairies with similar characteristics. In this study it was possible to observe that all farms studied are near surface watercourses (Figure 1) constituting a potential source of infection for cattle. For future studies it is recommended to consider the analysis and research of surface watercourses near these farms positive to infection by Cryptosporidium spp., in order to automate the necessary steps to prevent the spread of this disease among animals and its possible transmission to humans with the consequent health and economic damages.
In conclusion, it was determined that the frequency of infection by Cryptosporidium spp., in calves of dairy farms exceeds 50% in the Valdivia province using both the ZN stain and AU stain whose performance and concordance between the two tests was appropriate. Finally, it was possible to detect that the number of farms with cryptosporidiosis corresponded to 100% of the farms analyzed, observing a uniform distribution in the map associated with environments that are capable of sustaining a high production dairy.
Acknowledgments
This study was carried out thanks to the financing of the project DID UACH N° S-2010-19 and FONDECYT 1121035.
REFERENCES
1. Taylor M, Coop R, Wall R. Veterinary Parasitology. Oxford, United Kingdom: Blackwell; 2007. [ Links ]
2. Thompson R, Olson M, Zhu G, Enomoto S, Abrahamsen M, Hijjawi N. Cryptosporidium and Cryptosporidiosis. Adv Parasitol 2005; 59:78-139. [ Links ]
3. Chalmers R, Campbell B, Crouch N, Davles A. Clinical laboratory practices for detection and reporting of Cryptosporidium in community cases of diarrhoea in the United Kingdom. Euro Surveill 2008; 15:1-5. [ Links ]
4. Romero R, Pedrozo R, Vera E. La cryptosporidiosis en los terneros recién nacidos. Su etiología, patogenia, síntomas, tratamiento y profilaxis. Revista de ciencia y tecnología 2001; 3:99-108. [ Links ]
5. Díaz-Lee A, Mercado R, Onuoha E, Ozaki L, Muñoz P, Muñoz V, Martínez F, Fredes F. Cryptosporidium parvum in diarrheic calves detected by microscopy and identified by immunochromatographic and molecular methods. Vet Parasitol 2010; 176:139-144. [ Links ]
6. Muñoz P, Fredes F, Díaz-Lee A, Mercado R, Ozaki L. Detección de Cryptosporidium spp. en terneras de lecherías de la Región Metropolitana mediante Ziehl Neelsen y confirmada por inmunocromatografía y ensayo molecular. Arch Med Vet 2011; 43:111-116. [ Links ]
7. Fayer R, Xiao L. Cryptosporidium and Cryptosporidiosis. New York, USA. Ed CRC Press, 2007. [ Links ]
8. De Quadros R, Marques S, Amendoeira C, De Souza L, Amendoeira P, Comparin C. Detection of Cryptosporidium oocysts by Auramine and Ziehl Neelsen staining methods. Parasitol Latinoam 2006; 61:117-120. [ Links ]
9. Fredes F, Raffo E, Muñoz P. First report of Cryptosporidium spp. oocysts in stool of Adélie penguin from the Antarctic using acid-fast stain. Antarct Sci 2007; 19(4):437-438. [ Links ]
10. Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. Edward Island, Canadá. AVC Inc. 2003. [ Links ]
11. Hänscheid T, E Valadas. Diagnosis of Cryptosporidiosis using PCR or auramine O with LED fluorescent microscopy: Which end of the stick? Acta Trop 2009; 109:247-248. [ Links ]
12. Brook E, Christley R, French N, Hart C. Detection of Cryptosporidium oocyst in fresh and frozen cattle faeces: comparision of three methods. Let Appl Microbiol 2008; 46:26-31. [ Links ]