Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268
Rev.MVZ Cordoba vol.19 no.2 Córdoba May/Aug. 2014
ORIGINAL
Evaluation of the nutritive value of sugarcane residues inoculated with fungus Fomes sp
Evaluación del valor nutritivo de residuos de caña de azúcar inoculado con hongo Fomes sp
Alex Olivera D,1 M.Sc, Emilio Aranda I,1* Ph.D, Jesus Ramos J,1 Ph.D, Luis Vargas V,1 Ph.D, Juan Zaldivar C,1 Ph.D, German Mendoza M,2 Ph.D.
1Colegio de Postgraduados, Campus Tabasco. Periferico Carlos A. Molina, Km 3.5. Carretera Cardenas-Huimanguillo. H. Cardenas, Tabasco, Mexico C.P. 86500.
2Universidad Autonoma Metropolitana-Xochimilco Biotechnology Department, Xochimilco, DF. Mexico.
*Correspondencia: earanda@colpos.mx
Received: April 2013; Accepted: November 2013.
ABSTRACT
Objective. Improve the nutritional value of mechanized sugarcane residues inoculating the fungus Fomes sp. EUM1. Materials and methods. The fungus Fomes was inoculated according to a 0, 0.1, 0.2, and 0.3% (w/v) treatment and incubated at a temperature of 35°C for 7, 10 and 13 days. It was obtained DM, OM, CP, ash, NDF and ADF and the effective degradation of DM, NDF and ADF, with an experimental factorial design of 3X3 and a completely randomized design. The factors were growing days in an Erlenmeyer flask (7, 10, and 13) and inoculum percentage (0.1, 0.2 and 0.3). The data were analyzed with the SAS statistical package. Results. Statistical significance was found in the interaction of the fungus growing days by percentage of inoculum, in the variables: DM, CP and pH. The NDF and ADF factor differed in the percentage of inoculum. Effective degradation showed significant for the same type of interaction in all the variables studied. Conclusions. The inoculation of the fungus increased ADF degradation by only 0.2% of the inoculum percentage, without any effect on effective degradation due to the use of soluble fractions at the beginning of the incubation. It is considered that the degradation occurs in stages that are important to consider for determining treatments to maximize the beneficial effects of the fungus in terms of ruminant nutrition.
Key words: Enzymes, fermentation, ruminants (Source: DeCS).
RESUMEN
Objetivo. Mejorar el valor nutritivo de los residuos de cosecha mecanizada de la caña de azúcar inoculando el hongo Fomes sp. EUM1. Materiales y métodos. El hongo Formes se inoculó de acuerdo al tratamiento 0, 0.1, 0.2 y 0.3% (p/v), incubándose a una temperatura de 35°C durante 7, 10 y 13 días. Se obtuvo la MS, MO, PC, cenizas, FDN y FDA y la degradación efectiva de la MS, FDN y FDA; con un diseño experimental de tipo factorial 3X3, con un diseño experimental completamente al azar. Los factores fueron días de crecimiento en matraz Erlenmeyer (7, 10, 13) y porcentajes de inclusión (0.1, 0.2 y 0.3). Los datos se analizaron con el paquete estadístico SAS. Resultados. Se encontró significancia estadística para la interacción días de crecimiento del hongo por porcentaje de inóculo, en las variables MS, PC y pH. La FDN y la FDA presentaron diferencias para el factor porcentaje de inóculo. La degradación efectiva mostró significancia para el mismo tipo de interacción, en todas las variables estudiadas. Conclusiones. La inoculación del hongo aumentó la degradación de la FDA, únicamente al 0.2% de porcentaje de inclusión, sin un efecto sobre la degradación efectiva, debido a la utilización de fracciones solubles al inicio de la incubación. Se considera que la degradación se produce por etapas que son importante considerar para la determinación de tratamientos que maximicen los efectos benéficos del hongo en términos de la nutrición de rumiantes.
Palabras clave: Enzimas, fermentación, rumiantes (Fuente: DeCS).
INTRODUCTION
One of the fundamental problems in the feeding of ruminants in the tropics is the low availability of dry matter in pastures in the rainy and dry seasons; contrary to what occurs in the rainy season. In the state of Tabasco, Mexico, there are 29,112 Ha planted with sugar cane, of which 28,705 Ha are harvested, with a total production of 1,780,551 T of sugarcane and an average of 62.03 T/Ha (1). After the mechanized harvesting, the amount of residues (leaves, tips, stems, buds) remaining in the field is 18 T/DM/Ha (2), which could be used when there is a shortage of fodder. However, the main factors limiting the digestion of sugarcane residues in ruminants are: low protein content and high-fiber content (2).
Solid fermentation can be defined as a process where microorganisms grow on solid material with very low water levels. The material may be byproducts generated by agricultural and forestry practices (3-6). This type of fermentation improves certain nutritional features of agricultural yields when used as substrates (7). The species of the genus Fomes is mesophilic, since the optimal temperatures for its growth are between 20 and 36°C, very few species are heat-tolerant. The fungus Fomes sp. EUM1 is capable of growing in a temperature ranging from 20 to 40°C; however, its optimum growth temperature has been found to be 30°C (8).
In order to reduce the fibrous components and increase the digestibility of low-quality agricultural byproducts, biotechnological fermentation processes have been used with fungus that produces enzymes such as xylanases, laccases and cellulases (9,10). Therefore, the objective of this study was to improve the nutritional value of mechanized sugarcane residues by inoculating the fungus Fomes sp. EUM1.
MATERIALS AND METHODS
Study site. The study was carried out in the Food and Animal Science laboratories of Colegio de Postgraduados, Campus Tabasco, located in Cardenas, Tabasco, Mexico.
Substrate. The residue of the mechanized sugarcane harvest from a freshly cut channel of the variety CP 20-86 was used in town C-31 of Cardenas, Tabasco, Mexico. It is mainly composed of: buds, green leaves, pods and dry leaves. It was grinded in a Nogueira mill model DPM-500.1.2.4, powered by a petrol engine obtaining particles between 2 and 5 cm in diameter.
Inoculum. The fungus Fomes of the white patch Fomes sp. EUM1 was used, which has the potential to produce lignocellulolytic enzymes, which were provided by Universidad Autonoma Metropolitana, Campus Iztapalapa, Mexico DF.
Culture medium. Malt extract was used for the propagation of the macro myketos as a source of carbon and nitrogen extracted from yeast. The culture medium was prepared by dissolving the following components in distilled water: 40 g/L of malt extract, 3 g/L of yeast extract and 18 g/L of bacteriological agar (4). This was sterilized in an autoclave for 15 minutes at 120°C. The sterile medium was poured into Petri dishes, which were subsequently inoculated with mycelium in a disc of 0.6 cm in diameter. They were incubated at 30°C for 7 days.
Determination of the DM of the biomass. This was performed within 7 days of the growth of the mycelium in the culture medium, and the result is used, solely, for the determination of the amount of mycelia for inoculation per treatment. The biomass was separated from the agar by washing the Petri dish with distilled boiling water. Once the biomass was separated, it was placed in a paper filter (Whatman No. 541) and dried in a forced air stove SHEL LAB at 60°C until a constant weight was achieved. The value of the DM of the biomass was obtained by weight difference.
Cultivation in solid medium. Erlenmeyer flasks of 500 ml were used and 105 g of dry substrate (mechanized sugarcane residue), 315 ml of distilled water and 1.05 g of urea were adding 24 h before being inoculation (11,12). Subsequently, all flasks were sterilized in an autoclave (120°C, 15 psi, 20 min) and inoculated with the fungus in accordance with the treatment 0%, 0.1% (w/v), (1.05 g), 0.2% (w/v), (2.1 g), 0.3% (w/v), (3.15 g) of the substrate used. These were incubated at a temperature of 35°C for 7, 10 and 13 days.
Chemical analysis. The dry matter (DM) and crude protein (CP) were determined according to the methodology proposed by the AOAC (12), as well as by the fractioning of the fiber in neutral detergent fiber (NDF) and acid detergent fiber (ADF), using the methodology proposed by Van Soest (13). The variable OM was calculated by a difference of 100 - ash; the variable pH was measured taking 10 g of the residues from each flask and placing the same in a 250 ml Erlenmeyer flask, where 90 ml of distilled water was added and stirred for 30 min. Then, it filtered and the pH was immediately measured with a Conductronic pH 10 portable meter.
Effective degradation rate. For the determination of the effective degradation (ED) of the DM (DMED), OM (OMED), NDF (NDFED) and ADF (ADFED), at 96 h, a differential equations system was developed from the concepts of effective degradation described by Noguera (14) and indicated below:
dS/dt=-Degradation;
dSE/dt=-Effective_degradation-Passage; dD/dt=Degradation; dDE/dt=Effective_degradation;
Passage=kp*SE;
Degradation=kd*S;
Effective_degradation=kd*SE;.
With the initial values:
INIS =100-fSD; INISE=100-fSD;
INID=fSD; INIDe=fDE; fSD=50;
kd = 0.03; kp = 0.03
Where:
S=Substrate for the calculation of degradation;
SE=Substrate for the calculation of effective degradation;
D=Degraded material;
DE=Degraded material taking into account the passage rate (Effective degradation);
fSD=Soluble fraction;
INIx=Initial value of the variable of state x.
The model was adjusted for the estimation of the soluble fraction (fSD) and degradation rate (td) from the in situ degradation curves of each treatment subject to chemical analysis. The ED corresponded to the value of the variable with the same name at 96 h. To obtain the degradation curves nylon bags were incubated with the material to be evaluated and were taken out at 6, 12, 24, 48, 72 and 96 h using the methodology described by Noguera (14). The program used for the adjustment of the parameters, and the obtaining of the ED was the Berkeley Madonna v8.01 (15).
Experimental design and statistical analysis. For the variables of chemical composition (DM, OM, CP, NDF and ADF) and pH, a 3X3 factorial experiment was conducted; where the factor A, days of growth of the fungus, was evaluated at three levels: 7, 10, and 13 days; factor B, percentage of inoculum, was studied at three levels: 0.1, 0.2 and 0.3% (w/v). The combination of the two factors and their levels generated a total of nine treatments that were housed in a completely randomized experimental design with two replications. A variance analysis was performed for each of the variables of chemical composition according to the experimental design proposed and the following linear model:
Yijk=µ+αi+γj+πij+eijk; for i = 1, 2, 3; j = 1, 2, 3; k = 1, 2;
Where:
Yijk represents the observation at level i of factor A with level j of factor B and replication j;
µ is the general mean;
αi is equivalent to the fixed effect of level i of factor A;
γj is the fixed effect of level j of factor B;
πij is the effect of the interaction A X B;
and eijk is the random error.
Subsequently, for the interaction of the days of growth of the fungus (A) with the inclusion percentage (B) and the main effects that showed to be significant (p<0.05), a multiple comparison of means test was conducted using the Tukey method (16, 17). The information was processed using the statistical analysis software SAS version 9.3 and the procedures glm and mixed (17).
For ED (DMED, OMED, NDFED, and ADFED) at 96 h, a 3X3 factorial experiment was conducted; where the factor A, days of inoculation, was evaluated at three levels: 7, 10, and 13 days; factor B, inclusion percentage, was studied at three levels: 0.1, 0.2 and 0.3% (w/v). The combination of the two factors and their levels generated a total of nine treatments, which were housed in an experimental design of complete randomized blocks, where each block was conformed by an animal. For each of the ED variables, a variance analysis was performed according to the experimental design proposed and the following linear model:
Yijk=µ+αi+γ_j+δk+πjk+eijk; for i = 1, 2; j = 1, 2, 3; k = 1, 2, 3;
Where
Yijk represents the observation of animal or block i, at level j of factor A with level k of factor B;
µ is the general mean;
αi is equivalent to the random effect of animal i, γj is the effect of level j of factor A;
δk is the fixed effect of level k of factor B;
πjk is the effect of the interaction A X B;
y eijk is the random error.
Subsequently, for the days of inoculation interaction (A) by inclusion percentage (B) and the main effects that resulted significant (p<0.05), a multiple comparison of means test was conducted using the Tukey method (16). The information was processed using the statistical analysis software SAS version 9.3 and the procedures glm and mixed (17).
RESULTS
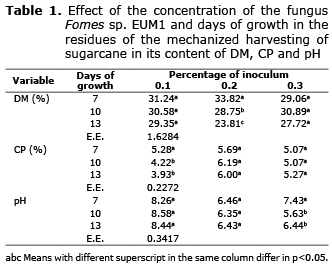
The results of the chemical analyses showed a statistical significance for the interaction A X B, days of growth of the fungus (A) by the inoculum percentage (B), in the variables: DM (p=0.0221), CP (p=0.0022), and pH (p=0.0170). The variables that showed statistical significance for factor A, days of fungal growth, were NDF (p=0.0082) and ADF (p=0.0185); while the variables OM and ashes were not significant (p>0.05) in all sources of variation. The DM only showed significant differences between the days of growth of the fungus of 0.2% (w/v) of the inclusion percentage, while CP and pH in 0.3% of growth. Table 1 shows the results of the variables with interactions. The global means of the variables were: DM=29.47, OM=90.58, CP=5.19, NDF=72.92, ADF=45.34, Ash=9.42 and pH=7.11. The NDF showed significant differences between day 7 (76.46%), day 10 (71.55%) and day 13 (70.75%) of incubation. Similarly, the ADF showed differences between day 7 (49.50%), day 10 (43.72%) and day 13 (42.81%).
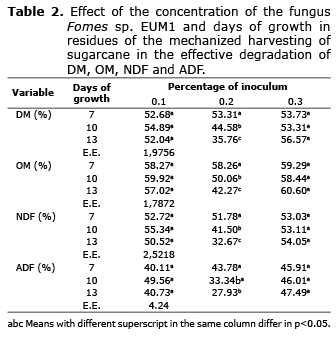
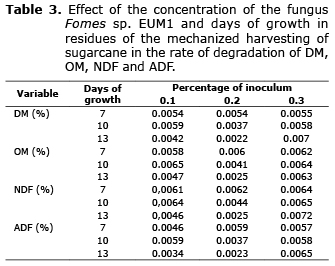
All variables which degradation was studied showed statistical significance for the interaction of the days of inoculation (A) and the percentage of inclusion (B), DM (p=0.0007), OM (p=0.001), NDF (p=0.0035) and ADF (p=0.0419). In all cases there were only interactions and significant statistical differences between the times of incubation of the inoculum at 0.2% (w/v) of the percentage of inclusion (Table 1), with an apparent decrease in the values of such variables as the days of incubation of the inoculum increased. The study of degradation kinetics showed that these declines were due to a reduction of td that went from 0.0054 to 0.0022, 0.0060 to 0.0025, 0.0062 0.0025 and 0.0059 to 0.0023, for, DM, OM, NDF and ADF (Table 2, 3), respectively, and not due to changes in the amounts of fSD, which showed marginal differences, except in the case of the ADF that was from 0.72 to 10.57% at day 7 to 13 of incubation. The means of all fSD were, 21.05, 26.24, 12.81 and 5.36%, for DM, OM, NDF and ADF, respectively.
DISCUSSION
The study of DM resulted in a pattern similar to that found in all variables where ED was studied (DM, OM, NDF and ADF). In these variables, differences were only found between the days of growth of the fungus at 0.2% (w/v) of the inclusion percentage, with a reduction of DM and ED as the days of incubation increased from day 7 to 13. In the case of the concentration of NDF and ADF, the same trend was observed, but for all percentages of inclusion. Similar results have been found in sugarcane bagasse treated with the strain Trichoderma viride M5-2, where a decrease in the NDF, ADF and Hc of 5, 3 and 2% were observed (18, 19). The decrease of DM could be due to the growth of the mycelium during the days of solid-state fermentation and synthesis of organic acids at the expense of a poor conversion efficiency from hemicellulose (Hc). The decrease in Hc as a result of the growth of the fungus has been previously reported in sugarcane silage treated with Pleurotus sapidus, although accompanied by an increase and not a decrease in DM (11).
The difference mentioned above may be due to the development phase of the fungus, the type of substrate used, the types of enzymes produced, the percentage of inclusion or an interaction of factors (20). This would explain why there were no differences between the days of incubation for the inclusion percentage of 0.1 and 0.3% (w/v) in DM and OM, as opposed to NDF and ADF. A too fast or too slow relative growth, as a consequence of the percentage of inclusion of the fungus, would leave outside the range of study sections where there are biological and statistical differences between treatments, since periods of stability can be found in all processes involving degradation, at both the beginning and end of these process. At the beginning because microorganisms require a time to adapt to the new conditions and at the end due to the end of the substrates (20).
For practical purposes, neither of these ends is suitable for the use of the fungus, since at the beginning it has not had time to produce enough enzymes to decrease degradation and at the end the substrate is already exhausted, they are not substrates that can be exploited by the ruminant. The significant differences found in concentrations of CP and pH at 0.1 and 0.3% (w/v) of inoculation, respectively, indicate the existence of processes different from those observed at 0.2% (w/v), and that the statistical similarities found between the levels of 0.1 and 0.3% (w/v) are a consequence of the final balance of the processes and not of identical compositions. Studies similar to this where specific chemical compounds such as Hc and true protein are evaluated could help to understand the changes that occur during the growth of the fungus.
In contrast to this study, in two experiments with the fungus Pleurotus sp., obtained from lignocellulose residues, the content of CP increased in 10-15% (21, 22). Likewise, Pal et al (9) reported an increase in CP in wheat straw fermented with the fungus Trametes versicolor and Pleurotus ostreatus.
Decreases in ED of observed in inclusion percentages of 0.2% (w/v) in all treatments (Table 2) could be caused, similarly for DM, and as it has been previously reported in experiments with Fomes sp. EUM1, because the fungus is using Hc as a source of energy for growth (22), before rising the production of enzymes capable of degrading lignin (22), with a peak of enzyme production of cellulases and xylanases between day 9 and 15 (9, 10). Since the types of enzymes found in the fungus under study are cellulases, xylanases and laccases (22), similar to those found in other studies (9, 10), this could indicate similar dynamics in the production of enzymes. In the same vein, Salcedo et al (23) reported an increase in the degradation of Hc in sugarcane residues treated with commercial ligninolytic enzymes previously using a delignification treatment.
Yan et al (24) found an increase in the in situ digestibility of DM (DMDI) in alfalfa and rice straw using exogenous enzymes. Similarly, in another experiment, an increase of DMDI was observed in a hay compound using 15 and 30% of the cellulose, xylanasa and fibrolytic enzymes (25), which contrasts with what was found in this study, since no increase of ED and/or td was found in any treatment (Table 2 and 3). Contrary to what was previously reported, the ED of DM (DMED) was reduced in 33% and td in 59%, when the inclusion percentage was 0.2% (w/v), and the incubation time increased from 7 to 13 days.
In the case of NDF, Membrillo et al (26) found an increase in the degradation of this fraction using the fungus Pleurotus ostreatus IE-8 and different substrate particle sizes, where the substrate was sugarcane bagasse. In alfalfa hay and rye grass treated with fibrolytic enzymes, Pinos et al (27) reported, in the same way, an increase in the in situ digestibility of NDF (NDFDI) and ADF (ADFDI) of 6 and 72 h. Likewise, in another study an increase in NDFDI was found using treatments with Saccharomyces cerevisiae and Aspergillus oryzae in alfalfa hay (28). However, in this study, both the NDF and the ADF showed a behavior similar to that previously described for DM. Therefore, only at a percentage of inclusion of 0.2% (w/v), the NDFDI decreased in 37% and td in 60%, while the ADFED, at that same percentage, dropped 36% and td 61%, when the incubation time increased from 7 to 13 days. The above represents something contrary to what was expected based on previously published reports. In this study, the decrease in DMED, OMED, NDFED y ADFED, in the only level of the inclusion percentage (0.2% w/v) where significant differences were reported, occurred as a consequence of the decrease in td as the days of incubation increased, since as mentioned above, changes in fSD were marginal (except for ADF) and that td showed marked decreases close to 60%.
In this study, the effect of the inoculation of the fungus, in terms of DM concentration, its fractions and degradation, could be due to the balance of the different processes that take place in each of the growth stages. It is possible that the level of 0.2% (w/v) of the percentage of inclusion, the use of easily degradable fractions, such as the Hc observed in other studies (11), could contribute to the decrease of the degradation of all fractions as the days of growth increased, since a fraction with high degradation is reduced leaving hard-degradation fractions. In this regard, the maximum time of growth of the fungus (13 days) could have limited the manifestation of enzymes on the fiber, since previous reports recommend up to 15 days (9,10) or more of fungal growth (11) to attain a significant effect. Even in these circumstances, the increase of 0.72 to 10.57% in the ADF’s fSD, found at a level of 0.2% (w/v) of the percentage of inclusion as the time of inoculation increased, is a clear indicator of the effect that the enzymes of the fungus have on the fiber, as well as of the need to increase the time of incubation for this effect to be reflected on ED.
Under the conditions in which this study was conducted, it is concluded that the inoculation of the fungus Fomes sp. EUM1 in mechanized harvesting of sugarcane waste has an effect on the ADF that is not reflected in the increase of degradation, due to the use of a highly degradable compound such as Hc, which is used as a source of energy at the beginning of its growth, although this effect only occurred at a level of 0.2% (w/v) of the inclusion percentage. Also, it is concluded that degradation occurs in stages that must be considered for the determination of the levels of the percentage of inclusion or incubation times where the beneficial effects of the fungus are maximized in terms of ruminant nutrition. Finally, the results obtained from the analysis of the ED of the different fractions of the material studied and their subsequent comparison with their corresponding fSD and td, demonstrated the usefulness of this methodology to study the processes that take place during the establishment, growth and production of enzymes by the fungus. The above must be taken into consideration for the planning of new experiments.
Acknowledgements
To the National Council of Science and Technology (CONACYT) for the scholarship granted for the study of the postgraduate studies on Agro-Food Production in the Tropics of Colegio de Postgraduados, Campus Tabasco. To UAM Ixtapalapa for its contribution of the strain that was used in this research. To the research lines of Colegio de Postgraduados LPI2 and LPI5 for the financial support for reagents and laboratory materials. The effective degradation model was developed for this study by Mr. Luis Manuel Vargas-Villamil.
REFERENCES
1. Sistema Integral de Información Agroalimentaria y Pesquera [en línea]. 2011. (acceso noviembre del 2013). URL Disponible en: http://www.siap.gob.mx/index.php?option=com_wrapper&view=wrapper&Itemid=351. [ Links ]
2. Lal R. Soil quality impacts of residue removal for bioethanol production. Soil Tillage Res 2009; 102:233-241. [ Links ]
3. Aranda EM, Ruiz P, Mendoza GD, Marcoff CF, Ramos JA, Elías A. Cambios en la digestión de tres variedades de caña de azúcar y sus fracciones de fibra. Rev Cubana Cienc Agric 2004; 38:137-144. [ Links ]
4. Fernandez JA, Henao JM, Pedrosa AM, Quevedo B. Inmovilización de hongos ligninoliticos para la remoción de colorantes negro reactivos. Rev Colomb Biotechnol 2009; 11:59-72. [ Links ]
5. Krishna C. Solid state fermentation systems: An overview. Crit Rev Biotechnol 2005; 25:1-30. [ Links ]
6. Arora DK, Bridge DP, Bhatnagar D. Handbook of Fungal Biotechnology. New York: CRC Press; 2004. [ Links ]
7. Peláez AA, Meneses M, Miranda RL, Megias RM, Barcena GR, Loera O. Ventajas de la fermentación sólida con Pleurotus sapidus en ensilaje de caña de azúcar. Arch Zootec 2008; 57:25-33. [ Links ]
8. Ordaz A, Favela E, Meneses M, Mendoza G, Loera O. Hyphal morphology modification in the thermal adaptation by the white rot fungus Fomes sp. EUM1. J Basic Microbiol 2011;52:167-174. [ Links ]
9. Pal M, Calvo AM, Terrón MC, González AE. Solid-state fermentation of sugarcane bagasse with Flammulina velutipes and Trametes versicolor. J Microbiol Biotechnol 1995; 11:541-545. [ Links ]
10. Sánchez A, Ysunza F, Beltrán M, Esqueda M. Cultivo del hongo comestible Pleurotus sobre residuos vitivinícolas y su manejo pos cosecha. [Tesis de Maestría]. Hermosillo, México: Universidad de Sonora; 2005. [ Links ]
11. Peláez A, Meneses M, Miranda A, Ayala M, Crosby M, Loera O et al. Enzimas fibrolíticas producidas por fermentación en estado sólido para mejorar los ensilajes de caña de azúcar. Agrociencia 2011; 45:675-685. [ Links ]
12. O.A.C. Official Methods of Analysis (18th Ed). Washington D.C.: O.A.C International; 2005. [ Links ]
13. Van Soest PJ, Robertson JP, Lewis BA. Symposium: Carbohydrate methodology, metabolism and nutritional implications in dairy caltle. J Dairy Sci 1991; 74:3583-3597. [ Links ]
14. Noguera RR, Posada SL. Modelación de la cinética de degradación de alimentos para rumiantes. Rev Col Cienc Pec 2007; 20:174-182. [ Links ]
15. Berkeley Madonna (programa de computadora). Versión 8.0. Berkeley: University of California; 2000. [ Links ]
16. Steel GDR, Torrie HJ, Dickey DA. Principles and procedures of statistics a biometrical approach. 3ra Ed. Michigan, USA: McGraw Hill Companies, Inc; 1997.
17. SAS/STAT® (programa de computadora). Versión 9.3. SAS Institute Inc; 2013. [ Links ]
18. Valiño EC, Elías A, Torres V, Carrasco T, Albelo N. Mejoramiento de la composición del bagazo de caña de azúcar por la cepa Trichoderma viride M5-2 en un biorreactor de fermentación en estado sólido. Rev Cubana Cienc Agric 2004; 38:145-153. [ Links ]
19. García Y, Ibarra A, Valiño EC, Dustet JC, Oramas A, Albelo N. Estudio de un sistema de fermentación sólida con agitación en la biotransformación del bagazo de caña de azúcar por la cepa Trichoderma viride M5-2. Rev Cubana Cienc Agric 2002; 36:265-270. [ Links ]
20. Arce O. Producción de extractos de enzimáticos a partir de Formes sp EUM1 y su evaluación en condiciones ruminales. [Tesis de Doctorado]. México: Universidad Autónoma Metropolitana, Unidad Iztapalapa; 2012. [ Links ]
21. Arias GM, Bueno G, Betancourt D, álvarez I, González AL. Biotransformación de Residuos Lignocelulósicos con Hongos Pleurotus. Rev CENIC Cienc Biol 2005; 36:1-7. [ Links ]
22. Akinfemi A, Ogunnwole OA, Lapido MK, Adu OA, Osineye OMES. Enhancement of the nutritive value of maize leaf treated with white-rot fungi: Pleurotus sajorcaju and Pleurotus pulmonarius, and the effects on chemical composition and in vitro digestibility. Prod Agric Technol 2009; 1:106-110. [ Links ]
23. Salcedo M, López J, Flores P. Evaluación de enzimas para la hidrólisis de residuos (hojas y cogollos) de la cosecha caña de azúcar. Dyna 2010; 78:182-190. [ Links ]
24. Yan H, Son Y, Beauchemin KA. Effects of exogenous enzymes on ruminal fermentation and degradability of alfalfa hay and rice straw. Asian-Aust J Anim Sci 2011; 24:56-64. [ Links ]
25. Giraldo LA, Carro MD, Ranilla MJ, Tejido ML. Influence of fibrolytic enzymes on in vitro methane production and rumen fermentation of a substrate containing 60% of grass hay. Livestock Research for Rural Development [en línea] 2007 (acceso 15 de noviembre del 2013); 19:Article 185. URL disponible en: http://www.lrrd.org/lrrd19/12/gira19185.htm
26. Membrillo I, Sánchez C, Meneses M, Favela E, Loera O. Particle geometry affects differentially substrate composition and enzyme profiles by Pleurotus ostreatus growing on sugar cane bagasse. J Microbiol Biotechnol 2010; 2:1581-1586. [ Links ]
27. Pinos J, González S, Mendoza G, Bárcena R, Cobos M. Efecto de enzimas fibroliticas exógenas en la digestibilidad in vitro de la pared celular de heno de alfalfa (Medicago sativa) o de ballico (Lolium perenne). Interciencia 2002; 27:28-32. [ Links ]
28. Miranda RLA, Mendoza MGD, Bárcena-Gama JR, González MSS, Ferrara R, Ortega CME et al. Effect of Saccharomyces cerevisiae or Aspergillus oryzae cultures and NDF level on parameters of ruminal fermentation. Anim Feed Sci Technol 1996; 63:289-296. [ Links ]