Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268
Rev.MVZ Cordoba vol.19 no.2 Córdoba May/Aug. 2014
ORIGINAL
Effects of dietary antioxidant of tomato extract and lycopene on Carassius auratus and Xiphophorus maculatus
Efecto antioxidante de un extracto de jitomate y licopeno en Carassius auratus y Xiphophorus maculatus
Cynthia Montoya M,1 IQ, Fernando Vega V,1 Ph.D, Héctor Nolasco S,2* Ph.D, Luis Espinosa C,1 M.Sc, Olimpia Carrillo F,3 Ph.D, Felicitas Olvera U,1 Lic. Biol.
1Universidad de Guadalajara. Centro Universitario de la Costa. Laboratorio de Acuicultura Experimental, Av. Universidad No. 203, Puerto Vallarta, Jalisco, México.
2Centro de Investigaciones Biológicas del Noroeste, Laboratorio de Fisiología Comparada, Instituto Politécnico Nacional No. 195, La Paz, B.C.S. México.
3Universidad de La Habana, Grupo de Biotecnología Marina, La Habana, Cuba.
*Correspondence: hnolasco04@cibnor.mx
Received: March 2013; Accepted: November 2013.
ABSTRACT
Objective. Evaluate the effect on tegument pigmentation, survival, growth and antioxidant capacity in diets supplemented with tomato extract and lycopene as additives in experimental feed for Carassius auratus and Xiphophorus maculatus. Materials and methods. The additives were added in different concentrations to a basic diet. We performed beginning and an ending biometrics for 100% of the population in each bioassay. The growth and survival of organisms were evaluated. The antioxidant capacity was analyzed by ABTS assay, both in the tomato extract sample as well as in foods used in different bioassays. The concentration of lycopene was determined in food and liver and muscle samples of fish fed with it. Acquired pigmentation of fish was assessed through photographs analyzed with Adobe Photoshop®. The results were evaluated by analysis of variance, and when differences were found (p<0.05) the means were compared by the Tukey test. Results. No significant effect (p>0.05) on pigmentation and growth of the organisms under the established experimental conditions was obtained. Significant differences in antioxidant capacity (p<0.05) were obtained in foods with added lycopene. Conclusions. The inclusion of lycopene or tomato extract in food for the organisms used is not recommended to improve pigmentation, but further studies are needed to demonstrate antioxidant effect.
Key words: Antioxidants, carotenoids, pigmentation, tomato (Source: CAB).
RESUMEN
Objetivo. Evaluar el efecto sobre la coloración de la piel, la sobrevivencia, el crecimiento y la capacidad antioxidante por dietas suplementadas con extracto de jitomate y licopeno como aditivos en la alimentación de Carassius auratus y de Xiphophorus maculatus. Materiales y métodos. Los aditivos se añadieron en diferentes concentraciones a una dieta base. Se realizó una biometría inicial y una final del 100% de la población en cada bioensayo. Se evaluó el crecimiento y supervivencia de los organismos. La capacidad antioxidante se analizó a través del ensayo ABTS, tanto a la muestra de extracto de jitomate como a los alimentos utilizados en diferentes bioensayos. Se determinó la concentración de licopeno a los alimentos y en muestras de hígado y músculo de peces alimentados con ellos. La pigmentación adquirida por los peces se evaluó a través de fotografías analizadas con el programa de Adobe Photoshop®. Los resultados fueron evaluados con análisis de varianza, cuando se encontraron diferencias (p<0.05) las medias fueron comparadas mediante la prueba de Tukey. Resultados. No se obtuvo un efecto significativo (p>0.05) sobre la pigmentación y el crecimiento de los organismos en estudio bajo las condiciones experimentales establecidas. Se obtuvieron diferencias significativas (p<0.05) en la capacidad antioxidante en los alimentos adicionados con licopeno. Conclusiones. La inclusión de licopeno o de extracto de jitomate en alimentos para los organismos utilizados no es recomendable con el fin de mejorar la pigmentación, aunque se requieren otros estudios para demostrar su efecto antioxidante.
Palabras clave: Antioxidantes, carotenoides, pigmentación, tomate (Fuente: CAB).
INTRODUCTION
In aquatic products, the color of skin and muscle is one of the main indicators of quality and affects the consumer's acceptance of the product and the price of the fish; for example, in salmon, color constitutes one of the most important criteria for quality when commercializing the product, in addition to fatty content and texture.
Carotenoids plays an important role in pigmentation of the skin and muscle color in fish. As in other animals, fish do not have the capacity to biosynthesize carotenoids, but must modify dietary carotenoids and store them in tegument and other tissues. Cultivated fish do not have access to a diet rich in carotenoids, and therefore it is necessary to add those compounds to their diet (1).
Due to the high cost and possible toxicity of synthetic pigments, the investigation in the use of yeast (2), marine bacteria, green algae (3), and plant extracts (4) as sources of natural pigment to give fish a bright color.
Carotenoids constitute one of the most important pigments in fish, and have a wide variety of functions including protection against adverse illumination, acting as a ProVitamin A, improving immune response, reproduction, growth, and maturity, regulation of chemotaxis in spermatozoids and is also a powerful antioxidants (5).
Lycopene is one of the first carotenoids that appear in the synthetic route, constituting of a molecular base for the synthesis of other carotenoids. Lycopene, which gives tomato its red color, is also a powerful antioxidant, combating oxidative stress and pathologies associated with it (6).
Carotenoids act as antioxidants due to their capacity to interact with oxygen reactive species, significant among them lycopene. Due to that, ingesting lycopene as an antioxidant in fish could help trap active oxygen species and reduce oxidative stress and the danger of oxidation of cellular components (lipids, proteins and DNA) (7); therefore, many functional foods are currently designed with the objective of providing an elevated amount of antioxidants and reducing the risk of diseases associated with oxidative stress (8).
This investigation was carried out to determine if the use of lycopene and tomato extract in diet could have a double function, as pigmentation as well as an antioxidant, affecting growth and survival of goldfish (C. auratus) and “platy” fishes (X. maculatus).
MATERIALS AND METHODS
Experiment site. The experiments took place in the Laboratorio de Acuicultura Experimental of the Centro Universitario de la Costa (CUCOSTA), belongs to the Universidad de Guadalajara, in Puerto Vallarta, Jalisco, Mexico. The cultivation water was taken from the municipal system, and left to sit (7 days) to eliminate the excess of chlorine.
Organisms and experimental units (EU). The fish used for the experiment were adult goldfish (Carassius auratus) and "platy" fish (Xiphophorus maculatus) from the installations of the Laboratorio de Acuicultura Experimental. In the EU the pH parameters were taken (7-8) (potentiometer HANNA Instruments®, Distrito Federal, Mexico), dissolved oxygen (7.4±0.4 mg/L) and temperature (25.1±2°C) (oximeter YSI®, Yellow Springs, OH 45387 USA) three times a week. The EU were kept well aired. Leftovers of food and fecal matter were cleaned daily. Sex determination was not performed.
Bioassays:
Bioassay with tomato extract. Seventy eight goldfish (25.9±6.8 g weight and 117.2±13.5 mm length) were distributed randomly in experimental units consisting of 400 L. circular tanks, with flow of filtered water in a filtration system with Aquadyne® spheres and a sterilizer ultraviolet light SmartUV®. Three organisms in each one of the 26 EU were used.
Bioassay with lycopene in C. auratus. Thirty six goldfish (37.8±10.8 g weight and 134.0±12.3 mm length) were distributed randomly in EU as described above. One was placed in each of the 36 EU.
Bioassay with lycopene in X. maculatus. Twenty three platys fish (0.3±0.1 g weight and 26.5±0.5 mm length) were distributed randomly in the EU, plastic boxes with 5 L. capacity. Supplementary air was supplied with an electric Boyu® aerator; every third day 100% of the water was changed to maintain clean water. A fish was placed in each one of the 23 EU.
Experimental diets and feeding. In all of the feeding tested in this study grenetina was used at 3% (w/w) as a binding agent to guarantee the adequate adhesion of the additives.
Bioassay with tomato extract. Goldfish were fed with Nutripec 2506 AP tilapia feed, Purina® brand, extruded, 3.5 mm length, and 25% protein. Tomato extract was added to the commercial feed (Lycopersicum esculentum) LICOPENE-100 made by Fórmulas Herbolarias Erick Estrada® in concentrations of 0, 1, 3 and 6% (w/w). The feed ration was calculated according to 4% of the initial biomass distributed in two feedings daily (9:00 and 14:00 h)for 45 days.
Bioassay with lycopene in C. auratus. The same feeding base was used as in the previous bioassay, adding lycopene made by Essential Nutrition® (50 mg of lycopene in, 75 mg of wheat germ per capsule) in concentrations of 0, 0.1, 0.3 and 0.6% (w/w). The feed ration was calculated according to 4% of the initial biomass distributed in two daily feedings (9:00 and 14:00 h)for 30 days.
Bioassay with lycopene in X. maculatus. Platys fish were fed with Nutripec 4510 H tilapia feed, Purina® brand, at spawning stage with 45% protein. The same commercial lycopene in the same concentration was added as described. Feeding was done ad libitum once a day (9:00 h) for 60 days.
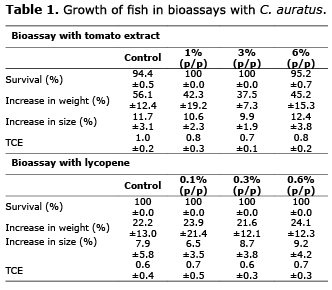
Experimental design. An initial and final biometry of 100% of the population was carried out, where the fish were weighed, measured and photographed in order to perceive the effect of the additives on the pigmentation and the parameters: survival, % weight increase, % length increase and specific weigh growth increase (9).
For bioassays with C. auratus, immediately after the final biometry 4 fish of each treatment were randomly selected and placed in a recipient with room temperature water. For the biotic management of stress in the fish, periodically (every 10 minutes) part of the water was substituted with cold water until the fish became lethargic by gradual hypothermia and were finally transferred to a freezer to finish killing them (-20°C, 16h). In this way the fish showed no evident stress behavior. The fish in the bioassay with tomato extract were cooked, submerged in distilled water for 3 minutes in a microwave, to boiling point; digital photographs were taken before and after cooking to evaluate pigmentation. Fish in the bioassay with lycopene were used to obtain samples of muscle and liver to determine the content of lycopene.
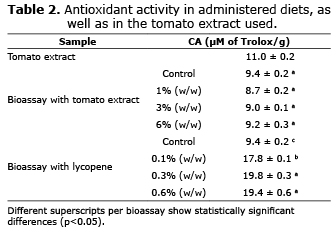
Determination of antioxidant capacity (CA). The feed in the bioassays of C. auratus were performed by the decoloration assay of the ABTS radical, according to the procedure described by Kuskoski et al (10). The initial resulting absorption of the radical ion ABTS was adjusted between 0.72-0.74 to a wave length of 734 nm (A734). After adding 50 µL of the extract of the sample to 1000 µL of the ABTS•+solution the A734 was brought to 37°C for 6 minutes. The results are expressed in µmol equivalents to Trolox/g.
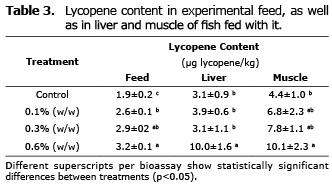
Lycopene determination. The bioassay with lycopene in C. auratus was done according to the method described by Fish et al (11) in the feed, as well as in the liver and muscles of the fish.
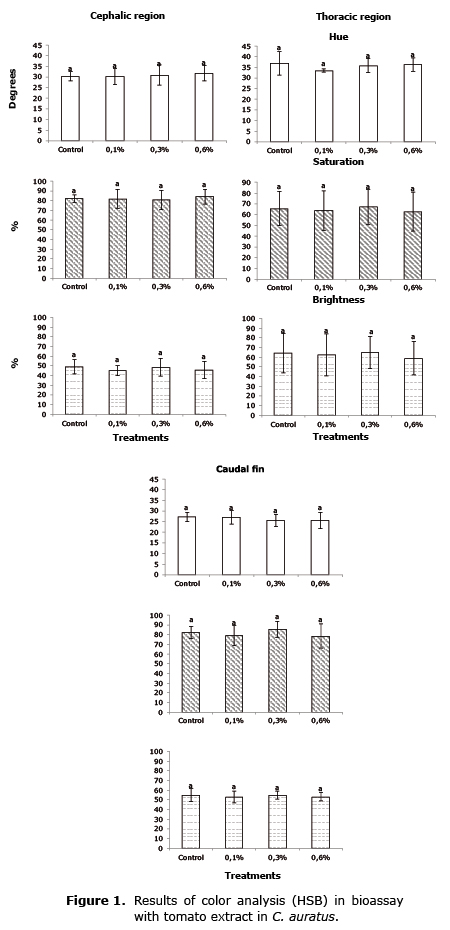
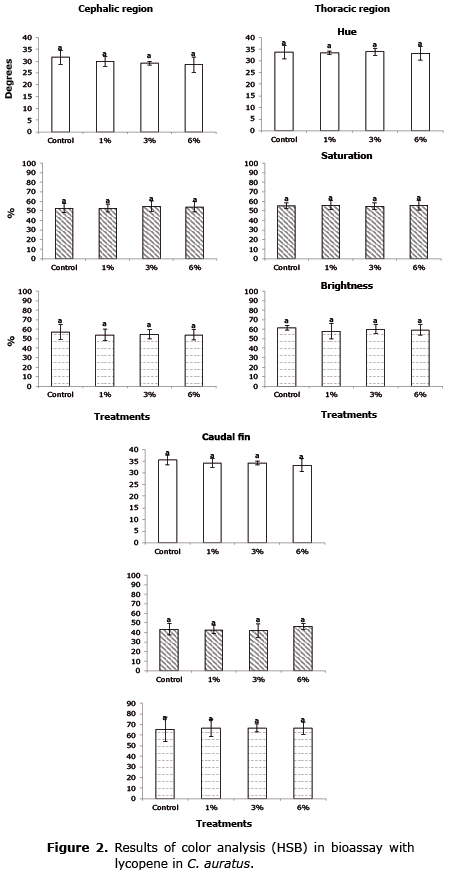
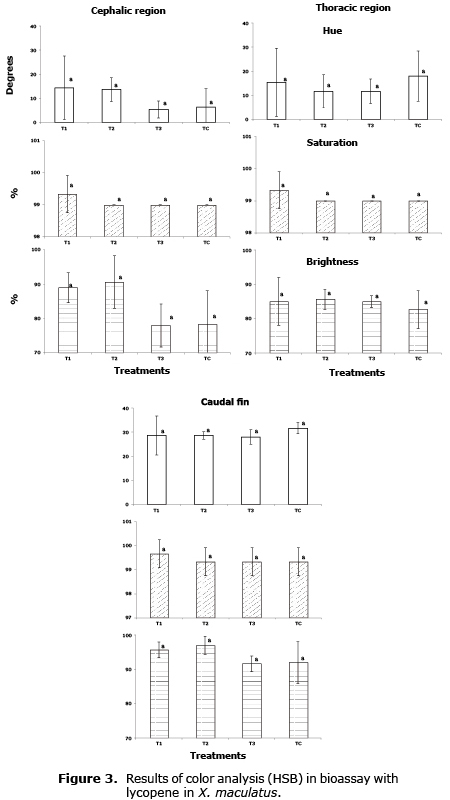
Pigmentation evaluation. The analysis of photographs was done with Photoshop® for specific regions of the body, according to the method described by Yasir et al (12). In C. auratus the cephalic, toracic and caudal fin region was analyzed; in X. maculatus the body, peduncle caudal and caudal fin. Values were obtained with the HSB model (Hue, Saturation and Brightness) to obtain possible changes in coloring.
Statistical analysis of data. The results obtained in this study were expressed as a measure of the values with their corresponding standard deviations. The results presented as percentages were transformed with a square root function of the Arcsine (13). Data from one experiment was statistically compared using the one way variation analysis (ANOVA) after normality tests (Kolmogorov-Smirnov; α= 0.05) and homocedasticidad (Bartlett; α= 0.05). The differences were considered statistically significant when p<0.05. The differences being statistically significant from the ANOVA, the differences between measurements were compared by means of the multiple measurements comparation test of Tukey. The data was analyzed with the SigmaStat V 3.1 statistics program (2004) (9).
RESULTS
The level of inclusion of tomato extract and comercial lycopene did not influence the growth of C. auratus (Table 1), since no significant differences were found (p>0.05) among the treatments, including the control group, in each one of the bioassays.
In the ABTS analysis of the C. auratus bioassays, no significant differences could be found (p>0.05) between the control and the feed with the tomato extract, but significant differences were found (p<0.05) in the feed with added lycopene, the control had lessened antioxidant capacity than the experimental feed (Table 2).
In determining the lycopene in experimental feed and the bioassay fish with lycopene in C. auratus (Table 3) significant differences were found (p<0.05), fish fed with the 0.6% treatment (w/w) had a greater capacity of accumulated lycopene in the liver.
The results of the analysis of the pigmentation were found in figures 1, 2 and 3. No significant effects (p>0.05) were found in the increase in pigmentation by means of the HSB analysis in any of the regions analyzed of the fish fed the experimental feed in any of the three bioassays.
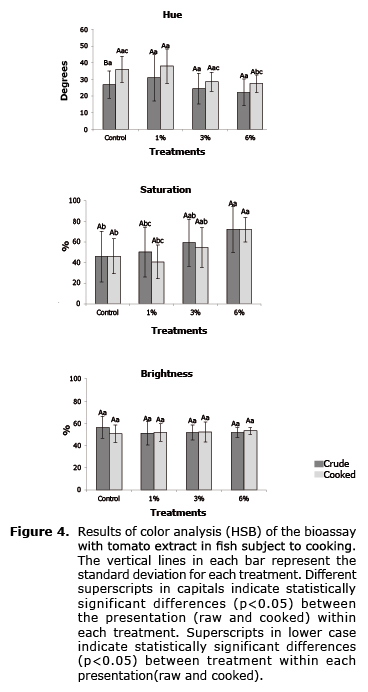
The red color of crustaceans is produced by carotenoid of the carotene-protein when it is denaturalized by cooking (14) so that this complex can affect the capacity to absorb light of the carotenoide molecule.
The analysis results of the pigmentation of raw and cooked fish in the bioassay with tomato extract in C. auratus is shown in figure 4. Significant differences (p>0.05) in the hue between treatments of the cooked fish are shown, but all treatments are the same as the control group, and therefore it could not be demonstrated that a connection exists between the supplied lycopene in diets with proteins in the tegument of the fish.
DISCUSSION
This study confirms that carotenoids do not play an important role in the growth of goldfish. Although these results agree with some studies, for example, Xu et al (2) reported that adding astaxanthin to red yeast (Xanthophyllomyces dendrorhous) did not have any effect on the weight gain in goldfish. Gouveia et al (15) performed two assays in the ornamental species of the goldfish (Carassius auratus) being fed a dietary supplement of carotenoids of the biomass of microalgae sweet water [Chlorella vulgaris, Haematococcus pluvialis, and cianobacteria Arthrospira maxima (Spirulina)], using a diet containing synthetic astaxanthin and a control diet, without coloring, for comparison, and it was found that feeding growth and efficiency were not significantly different among the groups with the different dietary treatments. The results of Ye şilayer et al (16) were similar, where young goldfish were fed five different diets: astaxanthin, canthaxanthin, Gammarus spp., paprika and a control group without supplement; it was found that growth and the feeding conversion index were not different from the control group in the groups fed with carotenoids.
Not all studies showed similar results: Kiriratnikom et al (3) reported that spirulina induces growth and color of goldfish; they found greater growth with a diet of 3% (p/p) of dried spirulina. Anhilan et al (17) in C. auratus (linnaeus) fed with botanical additives such as coriander, mint and amaranth as a source of carotenoids obtained better weight gain using amaranth at 1% (w/w), followed by fish fed by coriander at 3% (w/w). Wallat et al (18) used six different commercial diets supplemented with different carotenoids and showed they have a variable effect on skin color and the growth of juveniles of Oranda of C. auratus. Moreira et al (19) reported that in feeding treatments with Artemia and comercial feed in flakes in a circulation system and another with a static system, they found significant differences in growth (weight and size) in animals fed with Artemia (at 60 days). Also, they mentioned that the animals that received Artemia had improved pigmentation, although they did not offer any data on this last point.
The antioxidant capacity of experimental feed determined by the ABTS method has shown its increase in feed with added lycopene, but it could not be concluded that tomato extract does not increase the antioxidant capacity in the diets, since due to the complexity of the oxidation processes it is obvious that no one method of testing exists that completely reflects the antioxidant profile of the studied samples (20). Additionally, the antioxidant capacity of a mix is not just the sum of the antioxidant capacities of each one of its components; it also depends on the microenvironment in which it is found; the compounds interact with each other, which can produce synergic or inhibitor effects (10). Consideration should be given to the fact that the majority of the studies done that have been referred to consider the antioxidant activity of the additives or ingredients used, but generally do not evaluate the antioxidant effect of the diet as a whole.
Anhilan et al (17) comment that some investigators found that the development of color seems to depend on the quantity and nature of the carotenoid present in the source of the pigment/ingredient. This is confirmed by the present investigation, since no improvement was found in color when lycopene was used as a carotenoid, contrary to what was found in the majority of the studies that have evaluated the pigmentation of C. auratus with different natural sources of carotenoids and have shown to be an alternative to increase the pigmentation in these fish. In their investigation, Yanar et al (4) found that the addition of 15% alfalfa to the diet can guarantee good pigmentation and acceptable growth when used in goldfish.
Also, Gouveia et al (15) reported that microalgae (Chlorella vulgaris, Haematococcus pluvialis and Arthrospira máxima(Spirulina)) added as carotenoid to the diet increased the content of total carotenoids in the skin of goldfish and that good color was obtained using C. vulgaris and the best red color was obtained using H. pluvialis. Kiriratnikom et al (3) reported that the results of their investigation showed the positive effect of dried spirulina in the pigmentation of goldfish. Xu et al (2), when evaluating the addition of astaxanthin to red yeast (Xanthophyllomyces dendrorhous) in the diet of C. auratus, obtained an improvement in color; carotenoid deposits in fish fed with supplemented diets was significantly increased after the 15th day of feeding in comparison with fish fed with a diet without astaxanthin, but the effect on the color of the fish was evident on the 60th day. Sinha et al (21) made a similar observation in their study with Hibiscus rosasinensis petals, where they showed that it is a powerful natural source of carotenoids in C. auratus that improves their color as well as accelerates gonad development and growth.
Anhilan et al (17) confirmed that the percentage of color development was greater in fish fed with coriander than with amaranth and mint; this could be directly attributed to the higher level of carotenoid content. Orbe-Rogel (22) proved that adding carotenoids in the flower of petals of cempaxúchil presented effects in the growth rate and improved the pigmentation of the skin, although the high concentrations have a negative effect on the pigmentation of goldfish. Yeşilayer et al (16) demonstrated that paprika was as effective as a source of carotenoids as synthetic cantaxanthin and astaxanthin. The results indicated that the C. auratus fish were capable of using in an efficient manner the capsanthin paprika. Pérez-Escalante (23) observed that the food added with the pigments of ground Hibiscus flower can be used as an alternative source to the natural pigments to color C. auratus, as well as improving their growth.
Few studies have been done to evaluate lycopene as a compound with a pigmenting capacity in fish. Chatzifotis et al (1) reported that lycopene did not have significant effects on coloring the skin of red porgy (Pagrus pagrus); these results are in agreement with this study, where no significant effect on the pigmentation of C. auratus and X. maculates fish were found.
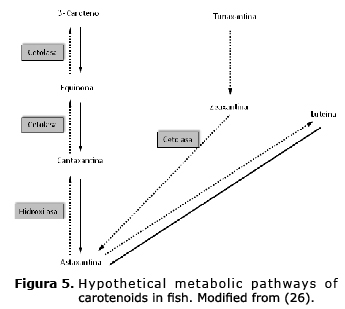
Carotenoids are synthesized by photosynthetic and carotenogenic organisms, the conversion of different carotenoids are catalyzed by enzymes (24,25). The primary red or carotenoid pigment in the skin of goldfish was identified as astaxanthin. Contradictory reports in literature exist about the capacity of goldfish to metabolize other carotenoid pigments in astaxanthin. Wallat et al (18), citing Hata et al (26), reported on the poor conversión of lutein and β-caroteno to astaxanth in goldfish, which is capable of metabolizing equinone to cantaxanthin but can barely metabolize β-carotene to equinone, suggesting that these results show the lack of enzyme in the first stage (Figure 5), which should be investigated in future studies.
Concerning lycopene, no investigation was found that could be used as a base to suggest the reason why fish used in this study could not incorporate pigments in their tegument from the lycopene.
With these results, it is proposed that fish show the capacity to absorb lycopene in the enterocytes of the intestinal wall, since it was found accumulated in muscles and liver in the treatment where they were fed at an inclusion level of 0.6%. This is congruent since the liver is the principal metabolic organ and excreter of carotenoids (27); however, it seems that this storage does not imply that the metabolisms of fish can achieved astaxanthin. The carotenoids were not found as complex carotene-protein in the tegument of the fish, as happens in invertebrate marine organisms, and that can affect the capacity of light absorption of the carotenoid molecule.
The results obtained suggest that the inability of lycopene to increase color in these fish can be due to the lack of an enzymatic system that transforms it into β-carotene and to astaxanthin, probably due to the lack of β-ciclase (25); however, this should be proved.
Based on the results of this investigation, it can be concluded that it is not recommended to include the extract of tomato or of lycopene in feed for Carassius auratus or for Xiphophorus maculatus in order to improve pigmentation and growth, although other studies are required to demonstrate the antioxidant effect in fish and its contribution to health improvement.
Acknowledgements
To Dr. José Luis Rodríguez of the Cuba Ministry of Food Industry. To M.C. Olimpia Chong Carrillo for providing information.
REFERENCES
1. Chatzifotis S, Pavlidis M, Doñate-Jimeno C, Vardanis G, Stereoti A, Divanach P. The effect of different carotenoid sources on skin coloration of cultured red porgy (Pagrus pagrus). Aquac Res 2005; 36(15):1517-1525. [ Links ]
2. Xu X, Jin Z, Wang H, Chen X, Wang C, Yu W. Effect of astaxanthin from Xanthophyllomyces dendrorhous on pigmentation of goldfish, Carassius auratus. J World Aquacult Soc 2006; 37:282-288. [ Links ]
3. Kiriratnikom S, Zaau R, Suwanpugdee A. Effects of various levels of Spirulina on growth performance and pigmentation in goldfish (Carassius auratus). Songklanakarin J Sci Technol 2005; 27:133-139. [ Links ]
4. Yanar M, Erçen Z, Özlüer HA, Büyükçapar HM. The use of alfalfa, Medicago sativa as a natural carotenoid source in diets of goldfish, Carassius auratus. Aquaculture 2008; 284(1-4):196-200. [ Links ]
5. Guillaume J, Kaushik S, Bergot P, Metailler R. Nutrición y alimentación de peces y crustáceos. España: Ed. Mundi-prensa; 2004.
6. Cardona EM, Ríos LA, Restrepo GM. Extracción del carotenoide licopeno del tomate Chonto (Lycopersicum esculentum). Vitae 2006; 13(2):44-53. [ Links ]
7. Waliszewski KN, Blasco G. Propiedades nutraceúticas del licopeno. Salud pública Méx 2010; 52(3):254-265. [ Links ]
8. Roberfroid MB. Global view on functional foods: European perspectives. Br J Nutr 2002; 88(Supl 2):S133-S138. [ Links ]
9. Vega-Villasante F, Martínez-López EA, Espinosa-Chaurand LD, Cortés-Lara MC, Nolasco-Soria H. Crecimiento y supervivencia del langostino (macrobrachium tenellum) en cultivos experimentales de verano y otoño en la costa tropical del pacífico mexicano. Trop Subtrop Agroecosyst 2011; 14(2):581-588. [ Links ]
10. Kuskoski EM, Asuero AG, Troncoso AM, Mancini-Filho J, Fett R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Ciênc Tecnol Aliment 2005; 25(4):726-732. [ Links ]
11. Fish W, Perkins-Veazie P, Collins J. A quantitative assay for lycopene that utilizes reduced volume of organic solvents. J Food Compost and Anal 2002; 15:309-317. [ Links ]
12. Yasir I, Qin JG. Effect of light intensity on color performance of false clownfish, Amphiprion ocellaris cuvier. J World Aquac Soc 2009; 40(3):337-350. [ Links ]
13. Zar JH. Biostatistical analysis. ed. 5ta. New Jersey, USA: Ed. Prentice-Hall; 2009.
14. Ponce-Palafox J, Arredondo-Figueroa J, Vernon-Carter E. Carotenoids from plants used in diets for the culture of the pacific white shrimp (Litopenaeus vannamei). Rev Mex Ing Quím 2006; 5:157-165. [ Links ]
15. Gouveia L, Rema P, Pereira O, Empis J. Colouring ornamental fish (Cyprinus carpio and Carassius auratus) with microalgal biomass. Aquacult Nutr 2003; 9:123-129. [ Links ]
16. Yeşilayer N, Aral O, Karsli Z, Öz M, Karaçuha A, Yağci F. The effects of different carotenoid sources on skin pigmentation of Goldfish (Carassius auratus). The Israeli J Aquacult - Bamidgeh. 2011; 63(IIC:63.2011.523).
17. Ahilan B, Jegan K, Felix N, Ravaneswaran. Influence of botanical additives on the growth and colouration of adult Goldfish, Carassius auratus (Linnaeus). Tamil Nadu J Vet & Animal Sci 2008; 4(4):129-134. [ Links ]
18. Wallat GK, Lazur AM, Chapman FA. Carotenoids of different types and concentrations in commercial formulated fish diets affect color and its development in the skin of the Red Oranda variety of Goldfish. N Am J Aquaculture 2005; 67:42-51. [ Links ]
19. Moreira RL, Da Costa JM, Teixeira EG, Moreira AGL, De Moura PS, Rocha RS and Vieira RHSF. Performance of Carassius auratus with different food strategies in wáter recirculation system. Arch Zootec 2011; 60(232):1203-1212. [ Links ]
20. Pérez RM, Vargas R, Martínez FJ, García EV, Hernández B. Actividad antioxidante de los alcaloides de Bocconia arborea. Estudio sobre seis métodos de análisis. Ars Pharmaceutica 2003; 44(1):5-21. [ Links ]
21. Sinha A. and Oyas AA. China rose (Hibiscus rosasinensis) petals: a potent natural carotenoid source for goldfish (Carassius auratus L.). Aquac Res 2007; 38(11):1123-1128. [ Links ]
22. Orbe-Rogel JC. Elaboración de dietas adicionadas con cempaxúchitl (Tagetes erecta L.) y su efecto sobre el desarrollo del pez japonés (Carassius auratus L.). [Tesis maestría]. México: Instituto Politécnico Nacional, Centro de Desarrollo de Productos Bióticos; 2012. [ Links ]
23. Pérez-Escalante V, Aguirre-Guzmán G, Vanegas-Espinoza PE, Del Villar-Martínez A. Effect of Anthocyanin’s Extract from flour of Roselle calyx (Hibiscus sabdariffa) on growth and pigmentation of Goldfish (Carassius auratus). Thai J Vet Med 2012; 42(1):107-111.
24. Farré G, Sanahuja G, Naqvi S, Bai C, Capell T, Zhu C, Christou P. Travel advice on the road to carotenoids in plants. Plant Sci 2010; 179(1-2):28-48. [ Links ]
25. Giuliano G, Tavazza R, Diretto G, Beyer P, Taylor A. Metabolic engineering of carotenoid biosynthesis in plants. Trends in Biotechnol 2008; 26(3):139-145. [ Links ]
26. Hata M and Hata M. Carotenoid pigments in goldfish, VI. The effect of acth and phenyl-thiourea on the discoloration and the carotenoids metabolism. Camp Biochem Physiol A Comp Physiol 1973; 46(1):95-101. [ Links ]
27. Rajasingh H, Øyehaug L, Våge D, Omholt S. Carotenoides en la dinámica de salmón atlántico. BMC Biology 2006; 4:10. [ Links ]