Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268
Rev.MVZ Cordoba vol.19 no.2 Córdoba May/Aug. 2014
ORIGINAL
Removal of lead, mercury and nickel using the yeast Saccharomyces cerevisiae
Remoción de plomo, mercurio y níquel utilizando la levadura Saccharomyces cerevisiae
Cherlys Infante J,1* M.Sc, Deniles De Arco R,1 M.Sc, Edgardo Angulo M,2 M.Sc.
1Universidad de Cartagena, Faculty of Pharmaceutical Sciences, Pharmaceutical Chemistry Programme. Av del Consulado, Calle 30 Nro. 48-152 Cartagena, Colombia.
2Universidad del Atlantico, Faculty of Basic Sciences, Chemistry Programme. Dirección Km 7 Antigua vía Puerto Colombia, Barranquilla, Atlantico.
*Correspondence: cinfantej@unicartagena.edu.co
Received: June 2013; Accepted: December 2013.
ABSTRACT
Objective. In this study the biomass of the yeast Saccharomyces cerevisiae was used to remove lead, mercury and nickel in the form of ions dissolved in water. Materials and methods. Synthetic solutions were prepared containing the three heavy metals, which were put in contact with viable microorganisms at different conditions of pH, temperature, aeration and agitation. Results. Both individual variables and the interaction effects influenced the biosorption process. Throughout the experimental framework it was observed that the biomass of Saccharomyces cerevisiae removed a higher percentage of lead (86.4%) as compared to mercury and nickel (69.7 and 47.8% respectively). When the pH was set at a value of 5 the effect was positive for all three metals. Conclusions. pH was the variable that had a greater influence on the biosorption of lead on the biomass of Saccharomyces cerevisiae. The affinity of the heavy metals for the biomass followed the order Pb>Hg>Ni.
Key words: Bioremediation, heavy metals, biomass, contaminant removal, bioaccumulation (Source: DECS).
RESUMEN
Objetivos. En este estudio se utilizó la biomasa de la levadura Saccharomyces cerevisiae para retener plomo, mercurio y níquel en forma de iones disueltos en agua. Materiales y métodos. Se prepararon soluciones sintéticas que contenían los tres metales pesados, las cuales se pusieron en contacto con el microorganismo en forma viable a diferentes condiciones de pH, temperatura, aireación y agitación. Resultados. Tanto las variables individuales como los efectos de interacción influyeron sobre el proceso de biosorción. A través de todos los experimentos, se observó que la biomasa de Saccharomyces cerevisiae eliminó un mayor porcentaje de plomo (86.4%) en comparación al mercurio y al níquel (69.7 y 47.8% respectivamente). Cuando el pH se fijó en valor de 5, el efecto fue positivo para los tres metales. Conclusiones. El pH fue la variable que tuvo una mayor influencia en la biosorción de plomo sobre la biomasa de Saccharomyces cerevisiae. La afinidad de los metales pesados por la biomasa siguió el orden Pb>Hg>Ni.
Palabras clave: Biorremediación, metales pesados, biomasa, remoción de contaminantes, bioacumulación (Fuente: DECS).
INTRODUCTION
Low concentrations of heavy metals can be highly toxic to microorganisms, whereas high concentrations can be tolerated if they are provided with enough sulfur. The toxicity of metals such as lead is well known; however, for others such as copper, zinc and nickel, their effects and permissible limits in surface waters are still under discussion (1).
Uptake mechanisms range from physical adsorption, ion exchange with the functional groups of the cell wall of the microorganism or they may penetrate into the cytoplasm and accumulate in the form of granules or intracellular inclusions (2).
Various microorganisms have been used for bioremediation purposes, such as bacteria, yeasts, fungi and microalgae; biosorbents based on macroalgae in non-viable state have also been prepared and have been effective in the removal of heavy metals from industrial effluents at concentrations ranging from 1 to 100 mg/L (2).
Growing cells may show a greater capacity for the removal of metals than non-viable biomass, especially in environments with enough nutrients (3)
Sources of pollution may be natural such as the erosion and weathering of rocks; or anthropogenic erosion, manufacturing pigments, batteries, biocides, processing of ores and metals, industrial effluent from electroplating plants, effluents from tanneries and sanitary landfills (4).
Saccharomyces cerevisiae possesses phosphate, amino, carboxyl and hydroxyl groups in its cell wall, which are responsible for the removal of heavy metals (5).
The advantages of the use of Saccharomyces cerevisiae include the global boom in bioethanol production, which ensures the availability of a steady supply of residual biomass that could be used in the bioremediation of industrial effluents (6). Moreover, this yeast is considered as safe, which favors its use in practical applications that will thereafter be easily accepted by the population. S. cerevisiae has also been immobilized for recovering precious metals such as platinum (7), being considered as a cheap and plentiful source of biomass, making its application feasible in the decontamination of effluents from the mining industry.
The brewing industry is another important source of low-cost yeast with flocculating properties, which facilitates the mechanical separation at the end of the biosorption process (8).
On the other hand, environmental conditions such as aeration, agitation, temperature and pH influence cell growth and an effect on the uptake of heavy metals associated with both the microorganism and the fluid dynamics of the medium could therefore be expected.
The adsorption of heavy metals in the biomass of S. cerevisiae has been reported in numerous studies (6-11).
Based on the above, experiments were carried out in order to determine the influence of the variables pH, temperature, agitation and aeration on the biosorption of lead, mercury and nickel in the biomass of S. cerevisiae.
MATERIALS AND METHODS
Microorganism. Commercial dry active baker’s yeast was used, which was kept in refrigeration at 4°C.
Culture medium. A culture medium containing 20 g/L of glucose, 20 g/L of peptone and 10 g/L of yeast extract was prepared for the cultures per lot of S. cerevisiae.
This medium was dosed with lead nitrate, nickel chloride and mercuric chloride solutions to obtain a concentration of 100 µM in each of the heavy metals, using a workload of 100 mL in each experiment.
Biosorption. Biosorption tests were carried out according to a 24 factorial design in which the levels of each factor were identified according to the coding (Table 1).
The tests were conducted by inoculating the medium with 0.2 g of dry yeast and incubated for 24 hours, to then separate the phases by filtration with a 0.45 µm paper.
Determination of heavy metals. The atomic absorption spectroscopy method was selected to analyze the heavy metals, using the spectrophotometer UNICAM 969. All samples were analyzed in triplicate.
The lead and nickel were determined using the flame atomization technique, while the cold vapor technique was used for analyzing mercury, on the basis of the standardized methods compiled in the APHA in section 3112B (12).
Experiment design. A complete 24 factorial design was used. The experiments planned through the combination of the variables under study were performed randomly and in duplicate.
The removal percentages for each metal were reported as the mean of the experiments ± the standard deviation of the mean effect, calculated from the effects of third and fourth order interactions.
The software SPSS Statistics version 17.0 was used for the calculation of the effects. The effects calculated were compared with a reference distribution (F test) at a confidence level of 95%.
RESULTS
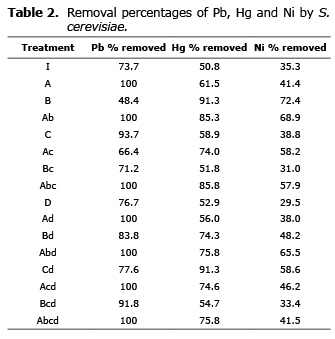
The biomass of S. cerevisiae adsorbed the metals under study in different proportions (Table 2), showing greater affinity for lead and lower affinity for nickel.
For average removed for lead was 86.4±3.8, for mercury 69.7±3.66 and for nickel 47.8±2.67. The average biosorption capacity for lead was 8.9 mg/g of dry yeast. The only metal for which a removal of 100% was obtained was lead, particularly in experiments with a pH of 5, except the trial where the agitation and pH were at a high level.
The biomass of S. cerevisiae showed an average capacity for mercury of 10.25 mg/g. The adsorption capacity for nickel was 2.8 mg/g of dry yeast.
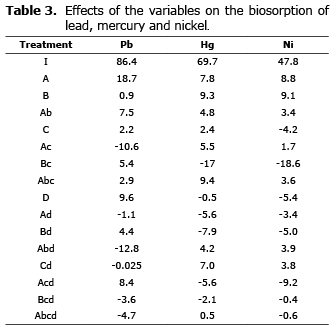
A significant influence was observed in the variables for each metal, represented by simple effects and second and third order interactions (Table 3). The effects calculated correspond to the coefficients of the linear model, which were considered significant when the likelihood value was less than 0.05 (p≤0.05).
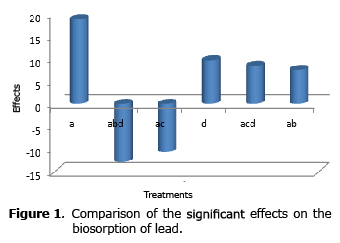
Analysis of the effects on the biosorption of lead. Of the six significant effects for this metal, the pH was the variable that had the greatest influence on the uptake of lead by the biomass (Figure 1).
The second most significant and favorable effect for the retention of lead was aeration, this result indicates that the viable state and oxidative metabolism of yeast influence positively on the uptake of this metal. The interaction between pH and agitation had a negative effect on biosorption.
In terms of third-order interactions, a favorable effect was observed for the interaction between pH, agitation and aeration, which indicates that if the biosorption process is mechanically agitated at a pH of 5, it also must be aerated in order to generate this positive interaction; while the interaction between pH, temperature and aeration was negative, setting the temperature as a critical variable in this process.
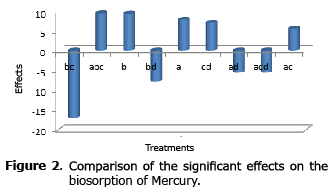
Analysis of the effects on the biosorption of mercury. As shown in Figure 2, most of the effects were significant for mercury biosorption: temperature, pH, all second-order interactions except for that between pH and temperature; as well as third-order interactions between pH, temperature and agitation and between pH, agitation and aeration.
In addition, a greater number of positive rather than negative effects was noted. The temperature and pH had positive effects as well as the interactions between pH and agitation as well as between agitation and aeration.
The interactions between temperature and agitation; temperature and aeration; as well as pH and aeration, showed a negative effect, the first being the stronger, indicating that the desorption of mercury is favored under these conditions.
The third-order interaction between pH, temperature and agitation was positive and had a strong effect comparable to the effects of pH and temperature separately on the uptake of mercury; while the interaction between pH, agitation and aeration had negative effect.
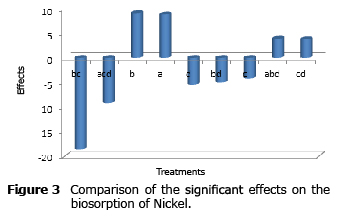
Analysis of the effects on the biosorption of nickel. Figure 3 shows that the biosorption of nickel displayed the highest number of negative effects, which is consistent with the lower removal percentage observed in this study.
All the variables under study influenced on the biosorption of nickel individually, temperature and pH with positive effects and agitation and aeration with negative effects but with a lower weight than the first two.
The most significant effect was the second-order interaction between temperature and agitation; it can be noticed that as in the case of mercury biosorption, it was negative and had a higher magnitude than the rest of the effects.
It is observed that the third-order interactions, which were significant for the biosorption of lead, were also significant in the case of nickel, but with the opposite sign.
DISCUSSION
The higher affinity of biomass for lead was a result in line with what has been reported in other studies (13, 14).
At a pH of 5, lead is a positive divalent ion that can interact with the negative functional groups of the cell wall of yeast (13).
Zhang et al (15) found an adsorption capacity of 6.52 Pb/g mg of dry yeast, lower to that obtained in this study (8.9 mg Pb/g of dry yeast). Skountzou et al (16) reported a capacity of 4 mg Pb/g based on a concentration of 20 mg/L, as that used in this study; in addition, these authors obtained a removal percentage for lead of 71.8%, lower to that obtained in this study.
Galedar and Younesi (17) reported a capacity of 1.2 mg Ni/g of dry yeast in experiments with S. cerevisiae immobilized at a pH of 8. Again, this capability was less than that obtained in this study. Another study found a removal rate for nickel of 72% from synthetic solutions, higher than that obtained in this study (18).
Suazo et al (19) found a direct relationship between the uptake of nickel and pH using S. cerevisiae var ellipsoideus; at a pH of 3.5, the adsorption capacity was 5.74 mg Ni/g of dry yeast for an initial concentration of 1000 µM. In this study, the capacity was approximately half of this value with an initial concentration ten times lower.
The repulsion between H+ ions and metal ions at a low pH restricts access to ligands, but as the pH increases, more negatively charged ligands are exposed to capture heavy metals (13).
The effect of aeration can be explained taking into account that biosorption is performed with cells in a viable state with an aerobic metabolism, it is expected that a good air supply is reflected in a specific high growth rate and a better use of the nutrients in the culture medium.
The effect of agitation is related to the shear forces generated that could cause the desorption of the metal joined by weak bonds such as electrostatic attraction, hydrogen bonding and Van der Waals forces.
The positive effect of temperature for mercury and nickel can be attributed to an increase in ionic mobility. The relationship between the metal removal capacity and temperature was studied by Zhang et al (15) finding that the maximum capacity was obtained at 30°C (20), which corresponds to the high level used in this study.
In conclusion, the pH was the variable that had the greatest influence on the biosorption of lead, mercury and nickel on the biomass of S. cerevisiae. The temperature was the variable that most influenced the biosorption of mercury and nickel. Lead was the metal with greater affinity for biomass, followed by mercury and nickel, which can be asserted by comparing their removal percentage and the effects of the interaction of temperature and agitation. Second and third order interactions were significant for the biosorption of lead, mercury and nickel. Aeration only had a positive effect on the biosorption of lead, which can indicate a relationship between the uptake of lead and the metabolic state of the cell. The strongest negative effect on the biosorption of mercury and nickel was represented by the interaction between temperature and agitation.
Acknowledgements
The authors would like to thank the Universidad de Cartagena for its support in the conduction of this study.
REFERENCES
1. Volesky B. Biosorption and me. Water Res 2007; 41:4017-4029. [ Links ]
2. Wang J, Chen C. Biosorbents for heavy metals removal and their future. Biotechnol Adv 2009; 27:195-226. [ Links ]
3. Li C, Xu Y, Jiang W, Dong X, Wang D, Liu B. Effect of NaCl on the heavy metal tolerance and bioaccumulation of Zygosaccharomyces rouxii and Saccharomyces cerevisiae. Biores Technol 2013; 143:46-52. [ Links ]
4. Soares E, Soares H. Bioremediation of industrial effluents containing heavy metals using brewing cells of Saccharomyces cerevisiae as a green technology: A review. Env Sci Pollut Res 2012; 19:1066-1083. [ Links ]
5. Gohari M, Hosseini S,Sharifnia S, Khatami M. Enhancement of metal ion adsorption capacity of Saccharomyces cerevisiae’s cells by using disruption method. J Taiwan Inst Chem E 2013; 44:637-645.
6. Wang J, Chen C. Biosorption of heavy metals by Saccharomyces cerevisiae: A review. Biotechnol Adv 2006; 24:427-451. [ Links ]
7. Mack CL, Wilhelmi B, Duncan JR, Burgess JE.Biosorptive recovery of platinum from platinum group metal refining wastewaters by immobilised Saccharomyces cerevisiae. Water Sci Technol 2011; (63):149-55. [ Links ]
8. Soares E, Soares H. Cleanup of industrial effluents containing heavy metals: a new opportunity of valorising the biomass produced by brewing industry. Appl Microbiol Biotechnol 2013; 97(15):6667-6675. [ Links ]
9. Ruta L, Paraschivescu C, Matache M, Avramescu S, Farcasanu I C. Removing heavy metals from synthetic effluents using “kamikaze” Saccharomyces cerevisiae cells. Appl Microbiol Biotechnol 2010; 85:763-771.
10. Li T, Liu Y, Peng Q, Hu X, Liao T, Wang H, Lu M. Removal of lead(II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: Kinetic and equilibrium modeling. Chem Eng J 2013; 214:189-197. [ Links ]
11. Ramírez M, Pereira M, Ferreira S, Vasco O, Ocampo C. Packed bed redistribution system for Cr(III) and Cr(VI) biosorption by Saccharomyces cerevisiae. J Taiwan Inst Chem E 2012; 43: 428-432. [ Links ]
12. APHA. Métodos normalizados para el análisis de aguas potables y residuales. Método 3112 B. 3-23, 3-24. 21ª Edición. España. Editorial Díaz de Santos; 2005.
13. Özer A, Özer D. Comparative study of the biosorption of Pb(II), Ni(II) and Cr(VI) ions onto S. cerevisiae: determination of biosorption heats. J Hazard Mater 2003; B100:219-229. [ Links ]
14. Chen C, Wang J. Influence of metal ionic characteristics on their biosorption capacity by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 2007; 74:911-917. [ Links ]
15. Zhang Y, Fan C, Meng Q, Diao Z, Dong L, Peng X et al. Biosorption of Pb2+ by Saccharomyces cerevisiae in Staticand Dynamic Adsorption Tests. Bull Environ Contam Toxicol 2009; 83:708-712. [ Links ]
16. Skountzou P, Soupioni M, Bekatorou A, Kanellaki M, Koutinas A, Marchant R et al. Lead(II) uptake during baker’s yeast production by aerobic fermentation of molasses. Process Biochem 2003; 38:1479-1482.
17. Galedar M, Younesi H. Biosorption of ternary cadmium, nickel and cobalt ions from aqueous solution onto Saccharomyces cerevisiae cells: batch and column studies. Am J Biochem Biotechnol 2013; 9(1):47-60. [ Links ]
18. Machado M, Soares E, Soares H. Removal of heavy metals using a brewer’s yeast strain of Saccharomyces cerevisiae: chemical speciation as a tool in the prediction and improving of treatment efficiency of real electroplating effluents. J Hazard Mater 2010; 180(1-3):347-53.
19. Suazo E, Morales L, Cristiani M, Cristiani E. Efecto del pH sobre la biosorción de níquel (II) por Saccharomyces cerevisiae var. ellipsoideus. Rev CENIC Cienc Quim 2010; 41:1-12. [ Links ]
20. Zhang Y, Liu W, Zhang L, wang M, Zhao M. Application of bifunctional Saccharomyces cerevisiae to remove lead(II) and cadmium(II) in aqueous solution. Appl Surf Sci 2011; 257:9809-9816. [ Links ]