Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268
Rev.MVZ Cordoba vol.19 no.3 Córdoba Sept./Dec. 2014
ORIGINAL
A new species of Schizopera (Copepoda: Harpacticoida: Miraciidae) from Colombia
Una nueva especie de Schizopera (Copepoda: Harpacticoida: Miraciidae) de Colombia
Juan M. Fuentes-Reinés,1* M.Sc, Samuel Gómez,2 Ph.D.
1 Universidad del Magdalena, Grupo de investigación en Limnología Neotropical. A.A 731 Santa Marta, Magdalena, Colombia.
2 Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Unidad Académica Mazatlán, Mazatlán, Sinaloa, México.
*Correspondencia: juanmanuelfuentesreines@yahoo.com
Received: March 2014; Accepted: June 2014.
ABSTRACT
Objective. The present contribution aims at the description of a new species of the genus Schizopera. Materials and methods. Water samples were collected in littoral areas with mangrove and macrophytes, and in the limnetic zone. Twenty five liters of water were taken. Water samples were filtered with a zooplankton net (45µm) and preserved in 70% ethanol. The filtered samples were concentrated to 100 ml and examined in a Bogorov camera. Copepods were separated. Observations and drawings of S. evelynae sp. nov. were made at a magnification of 1000X. Results. Schizopera evelynae sp. nov. seems to be closely related to S. giselae Jiménez -Álvarez 1988 and to S. pratensis Noodt 1958 based on the armature formula of P1-P4, but can be separated from these two species based on the relative length of P1ENP, length/width ratio of P1ENP2, relative length of the outer proximal and distal spines on P4EXP3, shape of the exopod and relative length of the exopodal setae of the female P5, shape and length/width ratio of the male P2ENP2, and male P5 baseoendopodal lobe:exopod length ratio. A key to the species of Schizopera from America is given. Conclusion. A new species of the genus Schizopera is described. The Colombian material shares most characters with S. giselae and S. pratensis.
Key words: Copepoda, distribution, harpacticoida, taxonomy, lagoon, new species (Source: CAB).
RESUMEN
Objetivo. Describir una nueva especie del genero Schizopera. Materiales y métodos. Muestras de agua fueron colectadas en áreas del litoral con mangle y macrófitas, y en la zona limnética. Se tomaron 25 L de agua. Las muestras de agua fueron filtradas con una red de zooplancton (45 µm) y preservadas en etanol al 70%. Las muestras filtradas se concentraron a 100 ml y fueron analizadas en una cámara de Bogorov. Los copépodos fueron separados. Se realizaron observaciones y dibujos de S. evelynae sp. nov. a un aumento de 1000X. Resultados. Schizopera evelynae sp. nov. parece estar estrechamente relacionada con S. giselae Jiménez-Álvarez 1988 y con S. pratensis Noodt 1958 basado en la fórmula de la armadura de P1-P4, pero puede ser separado de estas dos especies basado en la longitud relativa de P1ENP, relación longitud / anchura de P1ENP2, la longitud relativa de las espinas proximal y distal externas en P4EXP3, forma del exópodo y la longitud relativa de las setas del exópodo de la P5 de la hembra, la forma y la relación longitud / anchura del P2ENP2 del macho, y la relación entre la longitud del lóbulo baseoendopodal de la P5 y la longitud del exópodo en el macho. Se presenta una clave para las especies del género Schizopera en América. Conclusiones. Se describe una nueva especie para la ciencia del género Schizopera. Los especímenes colombianos comparten la mayoría de los caracteres con S. giselae y S. pratensis.
Palabras clave: Copepoda, distribución, harpacticoida, taxonomía, laguna, nueva especie (Fuente: CAB).
INTRODUCTION
Harpacticoida is one of the most important groups of meiobenthos. However, the study of this order from Colombian systems, especially those from estuarine environments, has been largely neglected.
Within Harpacticoida, Miraciidae is one of the most common and abundant families, being composed of about 364 species. Most of the species of Miraciidae are marine living forms inhabiting the benthic realm (1) and are distributed among 53 valid genera (2). Miraciidae contains two large genera, Schizopera (3) and Stenhelia (4) that account for almost 42 % of the whole family. The genus Schizopera comprises about 92 valid species and subspecies worldwide (5, 6), 18 of which have been described from the Americas (S. tobae tobae (7); S. haitiana(8); S. triacantha (8); S. vicina (9); S. gauldi (10); S. noodti (11); S. knabeni (12); S. californica (12); S. borutzkyi (13); S. anomala (14); S. carolinensis (14); S. tobae cubana (15); S. giselae. (16); S. pori. (16); S. chiloensis (17); S. osana (18); S. hawaiiensis (19); S. costaricana, (20)). Species of genus Schizopera can be found in marine, brackish and freshwater habitats (21, 22)
Apostolov (23) divided the genus Schizopera into three genera: Schizopera with two subgenera [Schizopera s. str. (23) and Neoschizopera (23)], Eoschizopera (24) with two subgenera [Eoschizopera s. str. (23) and Praeoschizopera (23)], and Schizoperopsis (23) with two subgenera [Schizoperopsis s. str. (23) and Psammoschizoperopsis (23)]. These groupings have been rejected by some authors (17,20,25). Mielke (17) united the genera Eoschizopera and Schizoperopsis into one genus, Schizopera.
The taxonomic position of the genus Schizopera is complex and controversial, and its ecology and phylogenetical relationships have been subject of discussions (17).
Following Mielke (17, 18), the subgenera created by Apostolov (23) and the genus Eoschizopera should not be accepted. Nevertheless, Karanovic (20) challenged Mielke's (17) view. Wells (26) agrees with Karanovic's (20) opinion but considers Schizoperopsis as synonym of Schizopera.
According to Karanovic & Cooper (5) the subdivision of the genus Schizopera must be now abandoned, being the only exception the genus Eoschizopera (22, 24) which includes four species (20). Thus, the generic division proposed by Apostolov (23) has also been abandoned (26).
The description of a new species of Schizopera and an identification key to the species of Schizopera from America is herein presented.
MATERIAL AND METHODS
Study area. Water samples were collected in Laguna Navío Quebrado (Los Flamencos Fauna and Flora Sanctuary, Camarones, La Guajira Department, Colombia) from April to December 2012 in littoral areas with mangrove and macrophytes, and in the limnetic zone.
Field methods. Twenty five liters of water were taken using a bucket of 25 L. The water samples were filtered with a zooplankton net (45 µm) and preserved in 70% ethanol.
Analytical methods. The filtered samples were concentrated to 100 ml and examined using a Bogorov camera. Copepods were separated and kept in 70% ethanol. Observations and drawings of S. evelynae sp. nov. were made at a magnification of 1000X from whole and dissected specimens mounted in lactophenol with a Leica compound microscope equipped with phase contrast and a drawing tube.
Museums and terminology. The type material was deposited in the Museo de Colecciones Biológicas de la Universidad del Atlántico - Colombia (UARC) and in the Copepoda collection of the Instituto de Ciencias del Mar y Limnología, Mazatlán Marine Station, Sinaloa state, Mexico (EMUCOP).
The terminology proposed by Huys & Boxshall (27) for the general description was adopted.
Taxonomical account
Family MIRACIIDAE Dana, 1846
Subfamily DIOSACCINAE Sars, 1906
Genus Schizopera Sars, 1905
Schizopera evelynae sp. nov.
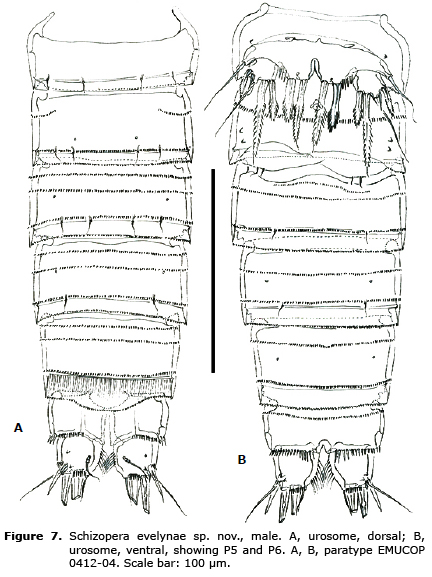
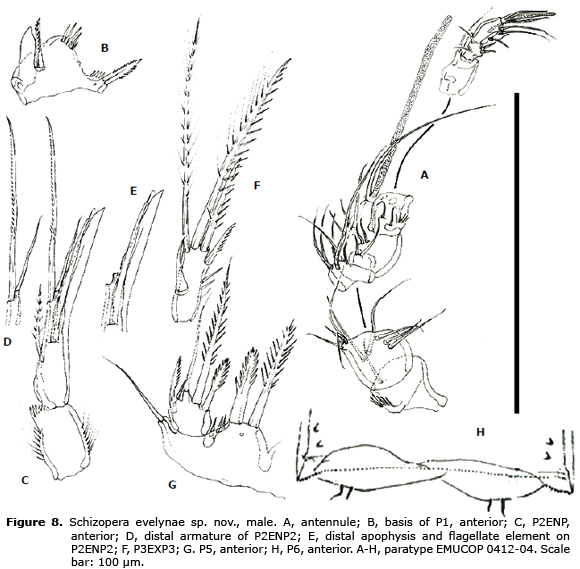
(Figures 1- 8)
Type material. One female holotype (UARC154M), one male allotype (UARC155M), and 16 female and 14 male paratypes (UARC156M) preserved in alcohol, and 29 female and 30 male paratypes preserved in alcohol (EMUCOP 0412-01) and 2 female (EMUCOP 0412-02, EMUCOP 0412-03) and 2 male (EMUCOP 0412-04, EMUCOP 0412-05) dissected paratypes; April to December, 2012. Leg. Juan M. Fuentes Reinés.
Type locality. Laguna de Navío Quebrado, Los Flamencos Fauna and Flora Sanctuary, Camarones, La Guajira Department, Colombia (11°25'N, 75°5' W). Salinity, from 0-28 PSU. Schizopera evelynae sp. nov. was found in the limnetic zone and in vegetated areas (mangroves); salinity, 28 PSU.
Etymology. The species is named in honor to Dr Evelyn Zoppy de Roa, for her work on zooplankton from Venezuelan systems and for her legacy and leadership of new generations of planktologists.
Description of female. Habitus (not shown) fusiform, tapering from the posterior part of the cephalothorax to the posterior part of the body. Total body length, measured from the tip of rostrum to the posterior margin of caudal rami, ranging from 532 µm to 602 µm (n= 11, mean= 567 µm). First urosomite (P5-bearing somite) without, posterior margin of genital double-somite, fourth and fifth urosomites with posterior finely serrated hyaline frill; dorsal hyaline frill of fifth urosomite bulging medially (Figures 1A). Genital double-somite fused dorsally and ventrally, with lateral rib indicating former division (Figure 1A, B); ventral surface plain, bearing P6, the latter represented by 1 outer short plumose seta and 1 inner long slender element (Figure 1B). Dorsal and ventral surface of genital double-somite, fourth and fifth urosomite with spinular pattern as figured (Figure 1A, B). Anal somite with semicircular smooth operculum (Figures 1A, C); with ventral spinules as shown (Figure 1D). Caudal rami as long as wide (Figures 1A-D); with 6 elements.
Rostrum not fused to cephalothorax, elongate, triangular, reaching tip of the second antennular segment (Figure 2A).
Antennule (Figure 2A) 8-segmented; armature formula: 1(1), 2(9), 3(7), 4(2+ (1+ae)), 5(2), 6(3), 7(4), 8(5+acrotek).
Antenna (Figure 2B-D). Allobasis with short rows of spinules along inner margin proximally and at base of exopod, with 1 abexopodal seta. Exopod 2-segmented; first segment with 1 slender plumose seta; second segment with 2 setae. Free endopodal segment with inner row of spinules, and armed with 2 lateral inner spines and 2 slender setae, and with seven distal elements (1 spine, 3 geniculate elements, 1 slender seta, and 1 b spinulose element fused to 1 slender setae basally).
Mandible (Figure 2E). Gnathobasis with bi-dentate pars incisiva, three spinules and 1 pinnate seta. Coxa-basis with 3 plumose setae distally. Exopod small, with 2 setae. Endopod much larger than exopod, 1-segmented, with 2 inner setae (one of them shorter), and 5 distal setae.
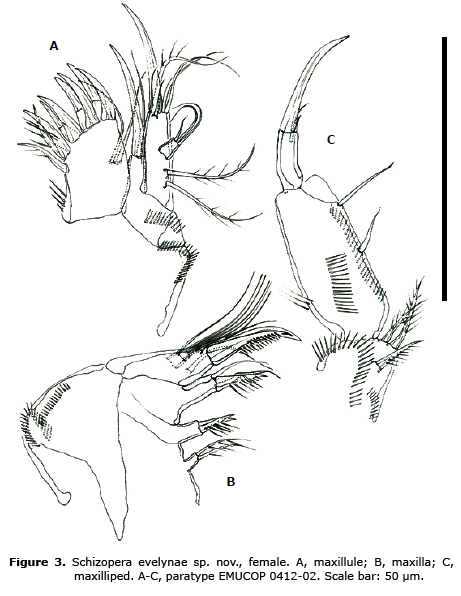
Maxillule (Figure 3A). Praecoxal arthrite with 8 b spines, 1 pinnate lateral element and 2 surface setae. Coxa with 1 b and 1 slender seta. Basis with 6 elements. Exopod fused to basis, with 2 setae. Endopod 1- segmented, with 3 slender setae.
Maxilla (Figure 3B). Syncoxa with three endites; first and second endites with 2 elements each, third endite with 3 elements. Endopod 2-segmented, with 6 setae.
Maxilliped (Figure 3C). Basis with spinules as shown and armed with 1 subdistal and 2 apical pinnate setae. Endopod 2-segmented; first segment with row of spinules along inner margin and 2 setae; second segment with b claw and 2 accompanying setae (1 of them very short).
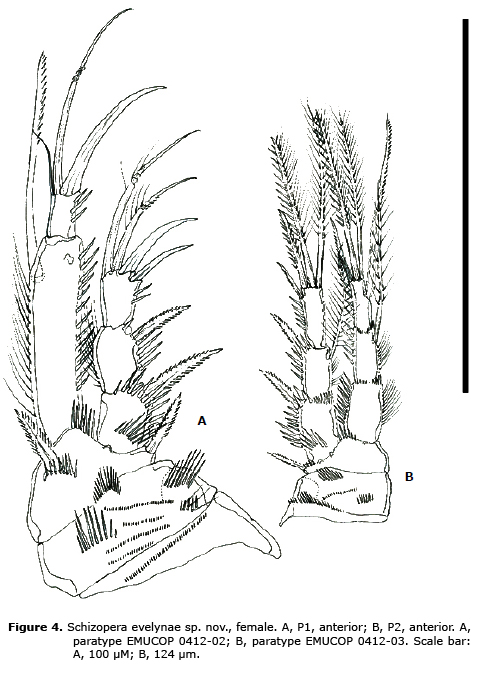
P1 (Figure 4A). Praecoxa, coxa and basis ornamented as shown. Basis with inner and outer spines. Exopod 3-segmented; exopodal segments without inner armature; EXP 3 with 4 elements. Endopod 2-segmented; first segment longer than exopod, about 4 times as long as wide, with a long inner seta subdistally; second segment about 1.6 times as long as wide, with one b spine, one geniculate element and one slender seta.
P2 (Figure 4B). Praecoxa, coxa and basis ornamented as figured. Basis with outer spiniform element. Rami 3-segmented. Exopod as long as endopod; EXP 1 and 3 without, EXP 2 with inner seta; EXP 3 with 4 elements. First endopodal segment without, second segment with 1 inner and 3 apical elements.
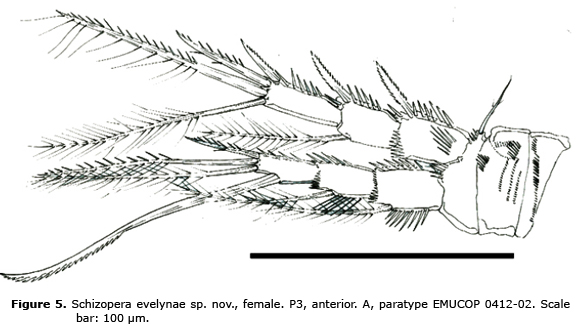
P3 (Figure 5). Spinular ornamentation of praecoxa, coxa and basis as shown. Basis with outer seta. Rami 3-segmented. Exopod as long as endopod; EXP 1 and 3 without, EXP 2 with inner seta, EXP 3 with 4 elements in all. ENP 1-2 with inner seta; ENP 3 with 1 b inner seta and 3 apical elements.
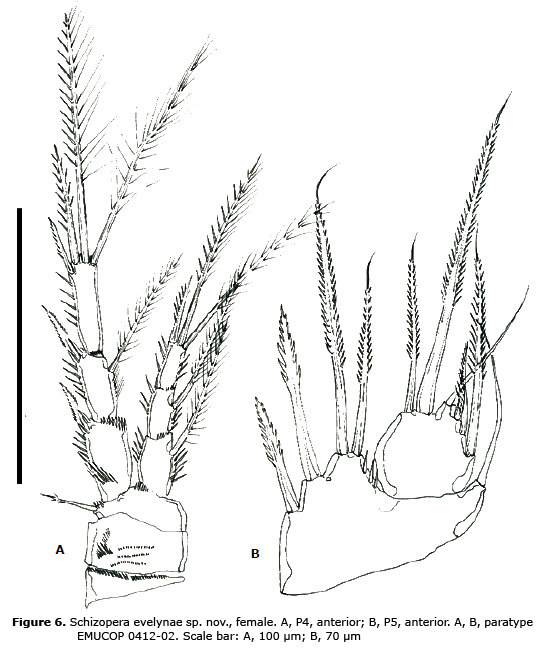
P4 (Figure 6A). Praecoxa, coxa and basis as in P3. Rami 3-segmented. Exopod noticeably longer than endopod; EXP 1-3 without, EXP 2 with inner seta; EXP 3 with 4 elements in all.
P5 (Figure 6B). Baseoendopodal lobe with 2 inner and 2 distal setae plus outer seta of basis. Exopod reaching beyond baseoendopod, with 6 setae.
Armature formula P1-P4 as follows:
Exopod Endopod
P1 I-0; I-0; I,I1,1 0-1; I11
P2 I-0; I-1;I,I1,1 0-0;0-1;I,2,1
P3 I-0;I-1;I,I1,1 0-1;0-1;I,2,1
P4 I-0;I-1;I,I1,1 0-1;0-1;I,2,0
Description of male. Habitus and rostrum (not shown) as in female. Total body length, measured from tip of rostrum to posterior margin of caudal rami, ranging from 400 to 420 µm (n=11; mean=410). Dorsal and ventral spinular ornamentation of urosomites (Figure 7A, B) as shown. Anal somite and caudal rami as in female.
Antennule (Figure 8A) 8-segmented, haplocer. First and fifth segment with spinules, second segment wide, third segment narrow, fourth segment swollen. Armature formula difficult to define, but most probably as follows: 1(1)-2(9)-3(6)-4(7+(1+ae))-5(2)-6(1)-7(4)-8(4+acrothek).
Antenna, mandible, maxillule, maxilla and maxilliped as in female.
P1 as in female except for dimorphic inner process of basis (Figure 8B).
P2 EXP (not shown) as in female. Endopod (Figure 8C) 2-segmeted, proximal segment without armature, distal segment modified as in figures 8D, E.
P3 as in female, except for the presence of a characteristic inner flattened hyaline spine on the third exopodal segment (Figure 8F).
P4 (not illustrated) as in female.
P5 (Figure 8G). Exopod with 5 setae. Baseoendopod with two b elements ornamented as figured, plus outer seta of basis.
P6 (Figure 8H). Each leg represented by one plate armed with 2 minute setae.
Variability. Female (11 females analyzed). The left P1ENP 2 of one female was observed to possess one instead of two setae; the left P1ENP 1 of one female lacks the lateral seta. The left P5ENP of two females possess five instead of four setae. Male (11 males analyzed). The right P2ENP 1 of one male was observed to possess a big spine instead of spinules.
Distribution and ecology. Schizopera evelynae sp. nov. is currently known from a single locality only, the protected coastal system Laguna Navío Quebrado, on the Caribbean coast of Colombia. This large (surface area of 10.7 km2) lagoon is a shallow water body (depth 0.3-1.1 m), whose temperature varies over the seasons from 28 to 31°C; pH values during sampling ranged between 7.8 and 8.3. In the surveyed area S. evelynae sp nov. was recorded in both the limnetic region and the vegetation zones (mangroves), being more frequent in the former habitat where salinity was highest (28 PSU). Fourteen species of Harpacticoida were reported in this water body (28).
DISCUSSION
Among the species of the genus Schizopera, S. evelynae sp. nov. seems to be closely related to S. giselae (16) and to S. pratensis (28) given the armature formula of P1-P4. However S. evelynae sp. nov. can be separated from S. giselae and S. pratensis by the relative length of P1Enp 1 (P1ENP 1 being longer that the exopod in S. evelynae, but as long as exopod in S. pratensis and S. giseale); length/width ratio of P1ENP 2 (about 1.4 in S. evelynae sp. nov. and 2.4 and 3 in S. giselae and S. pratensis, respectively). The anal operculum of S. evelynae sp. nov. is similar to that of S. pratensis, but differs from that of S. giselae in the setular ornamentation (with two rows of setules in S. giselae, but smooth in S. evelynae sp. nov. and S. pratensis). Also, the outer proximal and distal spines on the P4EXP 3 are different in length in S. evelynae sp. nov., and S. pratensis, but these outer spines are of the same length in S. giselae. The female P5 of S. evelynae sp. nov. is similar to that of S. giseale, but differs from that of S. pratensis in the shape of the exopod and in the relative length of the exopodal setae (exopod rounded in S. evelynae sp. nov. and S. giselae, but comparatively more elongate in S. pratensis).
The male of S. evelynae sp. nov. differs from the male of S pratensis in the length/width ration of P2ENP 2 (about 2.7 times as long as wide in S. evelynae sp. nov., but as long as wide in S. pratensis), in the shape of the apical elements on P2ENP 2 (one straight spine and three slender elements in S. evelynae sp. nov. but two long curved spines and one slender element in S. pratensis), in the length/width ration of the caudal rami (as long as wide in S. evelynae sp. nov., but 1.3 times as long as wide in S. pratensis), in the baseoendopodal lobe:exopod length ratio (longer than the exopod in S. evelynae sp. nov., but both rami of about the same length in S. pratensis).
Unfortunately, the male of S. giselae remains unknown and cannot be compared with the male of S. evelynae sp. nov.
In the following section, an identification key that might prove useful, at least, for preliminary identification of the valid Schizopera species for America, is presented. The key is based on female characters, because the male of S. giselae, S. triacantha, S. californica are unknown.

REFERENCES
1. Hendrickx ME, Fiers F. Copépodos Harpacticoida asociados con crustáceos decápodos. Cienc Mar 2010; 14:3-30. [ Links ]
2. Diversity and Geographic Distribution of Marine Planktonic Copepods [database on the Internet]. Banyuls-sur-Mer: Observatoire Océanologique de Banyuls, Université Pierre et Marie Curie (Paris VI) - CNRS/INSU (France). c2005-2014. [cited 2012 September 29]. [about 9p.]. Available from: http://copepodes.obs-banyuls.fr/en [ Links ]
3. Sars GO. An Account of the Crustacea of Norway. Volume V. Copepoda Harpacticoida, Parts 7-10. Plates XLVIII-LXXX, Bergen, Bergen Museum; 1905. [ Links ]
4. Boeck A. Oversigt over de ved Norges Hyster iagttagne Copepoder henhörende til Calanidernes, Cyclopidernes og Harpacticidernes Familier. Forh VidSelsk Kristiania 1865; 1864:1-57. [ Links ]
5. Karanovic T, Cooper S.J.B. Explosive radiation of the genus Schizopera on a small subterranean island in Western Australian (Copepoda:Harpacticoida): unraveling the cases of cryptic speciation, size differentiation and multiple invasions. Invertebr Syst 2012; 26:115-192. [ Links ]
6. World Register of Marine Species [database on the Internet]. Ostende: Flanders Marine Institute (Belgium). c2008. [cited 2014 January 20]. [about 18p.]. Available from: http://www.marinespecies.org/aphia.php. [ Links ]
7. Chappuis PA. Copepoda Harpacticoida der Deutschen Limnologischen Sunda-Expedition. Arch Hydrobiol 1931; Suppl 8:S512-584. [ Links ]
8. Kiefer F. Neue Ruderfusskrebse von der lnsel Haiti. Zool Anz 1934; 108:227-233. [ Links ]
9. Herbst HV. Copepoden (Crustacea, Entomostraca) aus Nicaragua und Südperu. Gewässer und Abwässer. 1960; 27:27-54. [ Links ]
10. Chappuis PA. Rouch R. Un nouvelle Nitocrella de Minorque. Arch Zool Exp 1961; 99:245-247. [ Links ]
11. Rouch R. Harpacticoïdes (Crustacés Copépodes) d'Amerique du Sud. In: C. Delamare Deboutteville & Rapoport E. (Ed), Biologie de l'Amerique Australe, Paris, Éditions du Centre National de la Recherche Scientifique; 1962. [ Links ]
12. Lang K. Copepoda Harpacticoidea from the Californian Pacific coast. Kungl. svenska VetenskapsakademiensHandlingar 1965; 10(2):1-560. [ Links ]
13. Montschenko V. Beitrag zur Kenntnis der Gattung Schizopera (Crustacea, Harpacticoida) im Schwarzen Meer Zool Anz 1967: 178:367-374. [ Links ]
14. Coull BC. Meiobenthic Harpacticoida (Crustacea, Copepoda) from the North Carolina continental shelf. Cah Biol Mar.1971; 12:195-237. [ Links ]
15. Petkovski TK. Subterrane Süsswasser-Harpacticoida von Kuba (Vorläufige Mitteilung). Résultats des expéditions biospéologiques cubanoroumaines à Cuba, Academiei Republicii Socialiste. Bucarest; 1973. [ Links ]
16. Jiménez-Álvarez MP. Harpacticoid copepods from Una do Prado River (São Paulo, Brazil): genus Schizopera. Hydrobiologia 1988; 167/168:435-444. [ Links ]
17. Mielke W. Description of some benthic Copepoda from Chile and discussion on the relationship of Paraschizopera and Schizopera (Diosaccidae). Microfauna Marina 1992; 7:79-100. [ Links ]
18. Mielke W. Species of the taxon Schizopera (Copepoda) from the pacific coast of Costa Rica. Microfauna Marina 1995; 10:89-116. [ Links ]
19. Kunz H. Schizopera hawaiiensis sp. n. (Copepoda, Harpacticoida) aus einer Lagune auf Oahu, Hawaii-Inseln. Mitt. Hamb Zool Mus Inst 1995; 92:65-72. [ Links ]
20. Karanovic T. Subterranean copepods from arid Western Australia. Crustaceana Monographs 2004; 3:1-366. [ Links ]
21. Apostolov A. Harpacticoïdes (Crustacea, Copepoda) de la mer Égée (plages de Kavala, Grèce du nord). Hist Nat Bulg 2008; 19: 5-33. [ Links ]
22. Karanovic T, McRae J. The genus Schizopera (Copepoda: Harpacticoida) in the Pilbara region of Western Australia, with a description of new species and its molecular and morphological affinities. Rec Aus Mus 2013; 28:119-140. [ Links ]
23. Apostolov A. Genres et sous-genres nouveaux de la famille Diosaccidae Sars et Cylindropsyllidae Sars, Lang (Copepoda, Harpacticoidea). Acta Zool Bulgar 1982; 19:37-42. [ Links ]
24. Wells, JBJ, Rao GC. The relationships of the genus Schizopera Sars within the family Diosaccidae (Copepoda:Harpacticoida). Zool J Linnean Soc 1976 58:79-90. [ Links ]
25. Bodin P. Catalogue of the new marine Harpacticoid Copepods (1997 Edition). Documents du travail de l`Institut Royal des Sciences Naturelles de Belgique. 1997. [ Links ]
26. Wells JBJ. An annotated checklist and keys to the species of Copepoda Harpacticoida (Crustacea). Zootaxa 2007; 1568:1-872. [ Links ]
27. Huys R, Boxshall GA. Copepod evolution. London: The Ray Society 1991; 113-152. [ Links ]
28. Fuentes-Reinés, J.M, Suárez-Morales, E. Annotated checklist and new records of Harpacticoida (Copepoda) from a coastal system of northern Colombia, South America. Crustaceana 2014; 87(2):212-255. [ Links ]
29. Noodt W. Schizopera pratensis n. sp. von Salwiesen der deutschen Meeresküste. Kieler Meeresforsch. 1958; 14:223-225. [ Links ]