Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268
Rev.MVZ Cordoba vol.19 no.3 Córdoba Sept./Dec. 2014
ORIGINAL
Determination of microbiological and sensory parameters of fish fillets with propolis preserved under refrigeration
Determinación de parámetros microbiológico y sensorial de filetes de pescado preservados con propóleos bajo refrigeración
Héctor Suarez M,1* Ph.D, Álvaro Jiménez T,2 M.Sc, Consuelo Díaz M,1 Ph.D.
1Universidad Nacional de Colombia. Instituto de Ciencia y Tecnología de Alimentos-ICTA. Carrera 30 N° 45-03 Edificio 500 C. Cundinamarca, Bogotá.
2Universidad Nacional de Colombia. Postgrado en Ciencia y Tecnología de Alimentos, Carrera 30 N° 45-03 Edificio 500 C. Cundinamarca, Bogotá.
*Correspondence: hsuarezm@unal.edu.co
Received: July 2013; Accepted: November 2013.
ABSTRACT
Objective. To determine the ability of propolis preservative cachama fillets during refrigerated storage. Materials and method. Ethanol extracts of propolis (EEP) were used in cachama fillets. The treatments included: i) 96% ethanol alcohol as the control; ii) 0.8% EEP; iii) 1.2% EEP; and iv) liquid smoke. A in vitro analysis was used to determine the inhibitory effect of propolis on Staphylococcus aureus, Escherichia coli, Salmonella sp. and Clostridium sp. and on the fish matrix to determine the mesophiles, psychrotrophiles, total coliforms, fecal coliforms, sulphite reducing spores and the presence of Salmonella. Results. The results of the in vitro analysis demonstrated the control that the EEP had over the evaluated microorganisms without presenting significant differences between the different concentrations (p>0.05). The analyses of the fish fillet matrix presented acceptable contents for the evaluated microorganisms in the treatments with EEP. A different situation was seen in the treatment with liquid smoke and the control, which had samples that where rejected after 20 days of storage. The sensory analysis showed acceptance for the samples with EEP until the end of the storage period but low marks for the treatment with liquid smoke and the control. Conclusions. The EEP used in this study could be effective for the control of Gram positive bacteria and some Gram negative bacteria that are present in cachama fillets; and could be an alternative to the use of chemical preservatives.
Key words: Cachama, ethanol extracts, microorganisms, preservation, Piaractus braquipomus (Sources: UNESCO, FAO).
RESUMEN
Objetivo. Determinar la capacidad conservante de propóleos en filetes de cachama durante el almacenamiento bajo refrigeración. Materiales y método. Se utilizaron extractos etanólicos de propóleos (EEP) en filetes del pez cachama (Piaractus brachypomus). Los tratamientos realizados fueron: i) alcohol etanólico 96% v/v como control; ii) EEP 0.8%; iii) EEP 1.2% y iv) Humo líquido. Fueron realizados análisis in vitro para determinar la actividad inhibitoria de los propóleos frente a Staphylococcus aureus, Escherichia coli, Salmonella spp., y Clostridium sp., y en la matriz de pescado para determinar la presencia de mesófilos, psicrotrófilos, coliformes totales, coliformes fecales, esporas sulfitoreductoras y de Salmonella spp. Resultados. Los resultados de los análisis in vitro mostraron control de los EEP sobre los microorganismos utilizados sin presentar diferencias significativas (p>0.05). Los análisis en la matriz del filete de pescado presentaron conteos aceptables para los microorganismos evaluados en los tratamientos con EEP. Situación diferente para los tratamientos con el humo líquido y el control, siendo las muestras rechazadas a partir del día 20 de almacenamiento. El análisis sensorial mostró aceptación de las muestras con EEP hasta el final del período de almacenamiento y bajas puntuaciones para los tratamientos con el humo líquido y el control. Conclusiones. Los EEP utilizados podrían ser efectivos en el control de bacterias Gram positivas y algunas Gram negativas presentes en filetes de cachama y serian una opción para evitar el uso de conservantes químicos.
Palabras clave: Cachama, conservación, extractos etanólicos, microorganismos, Piaractus brachypomus (Fuentes: UNESCO, FAO).
INTRODUCTION
The cachama fish (Piaractus brachypomus) hails from different basins of South America. In Colombia is the main native species in aquaculture. On the other hand, propolis is a natural, resinous substance and a strong adhesive, which is collected by Africanized honeybees (Apis mellifera L.) from leaf buds of trees and plants, and mixed with pollen and enzymes that are secreted by the bees (1). The bees use propolis as a sealant for the interior walls of hives and as a protective barrier against intruders (2). In general, propolis is composed of balsam and vegetative resin (50% v/v), wax (30% v/v), essential herb oils (10% v/v), pollen (5% v/v) and other substances (5% v/v), including organic remains (2). In ethanol extraction, the wax and organic residues are removed during the process. The ethanol extract that is obtained has more than 300 bioactive compounds, such as polyphenols, terpenoids, steroids, sugars and amino acids that have been detected in raw propolis.
Propolis is considered responsible for the lower incidence of bacteria and fungi in hives. The action against microorganisms is an essential characteristic of propolis. Furthermore, humans have used propolis for centuries due to the pharmaceutical properties (1). The antimicrobial biological activity is due to the presence of flavonoid and phenol compounds, although the exact action of this mechanism is unknown (3). The antimicrobial effect of propolis depends on the propolis extract concentration and is influenced by the extraction method (4). In addition, studies have shown that allergic reactions are not slightly common and that propolis is relatively no toxic, with effect values not observed (NOEL) in 1400mg/kg of body weight/day in mice (2).
A number of studies have mainly described the antimicrobial action against microorganisms (5, 6). The antibacterial effect of propolis on pathogens transmitted by food, such as Bacilluscereus, Staphylococcus aureus, Listeria monocytogenes, Enterococcus faecalis and Perfringenes Clostridium, has shown a potential for preserving food against such pathogens (7,8). In addition, propolis contains actions that not only counteract bacteria and fungi but also viruses. Drago and De Vecchi (5) reported that propolis is capable is inhibiting the growth of influenza viruses, adenoviruses, parainfluenza viruses, and the Herpes simplextype-1 virus.
In food applications, propolis has been proposed as a preservative for meat products due to the antimicrobial effect (9) and as a bactericide and insecticide in food packaging (10). Furthermore, it has been reported that the addition of propolis doubles or triples the shelf-life of frozen fish (8). In addition, the liquid smoke has antimicrobial activity, but must be used under certain thermal conditions.
In considering safety factors, it has been reported that various propolis compounds are present in nutritional and/or food additives and are considered GRAS (Generally recognize as safe) (2). These characteristics make propolis an attractive candidate for natural preservation in new food applications and able to satisfy the demand for antioxidants and antimicrobials that comes from the growing awareness of consumers for natural food and the omission of processing and chemical preservatives (9, 11). This study aimed to evaluate the antimicrobial and sensory capabilities of ethanol extracts of propolis applied to cachama fillets during a period of refrigerated storage.
MATERIALS AND METHODS
Ethanol extract of propolis (EEP). Propolis from the Villa de Leiva, Boyacá (Colombia) was utilized in this study. Twenty five % (v/v) ethanol extract of propolis in 96% (v/v) ethanol was used. Hundrey g of propolis were introduced in a precipitated glass, then 400 ml of ethanol 96% (v/v) were added. The mixture was shaken during two hours, and it was left at rest overnight, and then it was filtered. The residue was subjected to a secondary extraction with the same proportions as the first one. Finally the two extracts were mixed and frozen to precipitate other compounds. The supernatant EEP was used for the tests and its solid concentration of 8% (v/v) was established for the oven-drying method.
Preparation of the fillets. Transversal cuts were used on the cachama fillets to cut intramuscular bones. The following treatments were used: i) Control, 96% (v/v) ethanol alcohol; ii) propolis at a concentration of 0.8mg/ml; iii) propolis at a concentration of 1.2 mg/ml; iv) liquid smoke. The fillets were packed in vacuum sealed bags and stored at 3oC for 24 days.
Microbiological Analysis. The following microbiological analyses were carried out:
In vitro tests. Staphyloc occus aureus, Escherichia coli, Salmonella sp. and Clostridium sp., strains were obtained from the Microbiology Laboratory at the Instituto de Ciencia y Tecnología de Alimentos - ICTA at the Universidad Nacional de Colombia, Bogotá. For the activation of Clostridium sp., BHI broth (Oxoid) was used; for Salmonella sp., a nutritive broth (Oxoid) according to ATCC 13076; and for Staphylococcus aureus, Escherichia coli, TSB broth (Merck) according to ATCC 25923 and ATCC 25922, respectively. The strains were incubated overnight after 18 hours of growth.
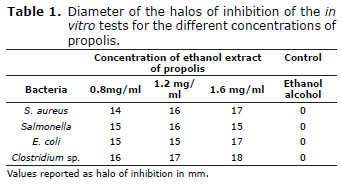
For the analysis of the inhibitory activity of propolis, a diffusion test was carried out in Mueller-Hinton agar. One ml of each strain was grown using 0.5 in the MacFarland scale, corresponding to a concentration of 1.5 x109 cells on the surface. Six mm diameter of filter paper discs were impregnated with three different propolis concentrations (0.8, 1.2 and 1.6 mg/mL) and a control with 96% (v/v) ethanol alcohol was used. They were incubated for 48 hours and the inhibition halos were measured.
It was found that the three concentrations inhibited the growth of the utilized pathogens and that there were no significant differences for the inhibitory effect, so the two lower concentrations were selected (0.8 and 1.2 mg/mL).
Cachama fillet tests. The plate mesophile (heterotrophs), psychrotrophile, total coliform, fecal coliform, and sulphite reducing spore counts and the presence of Salmonella spp., were determined according to the standards of the Normas del Instituto Nacional de Vigilancia de Medicamentos y Alimentos- INVIMA and Normas Técnicas Colombianas (12).
Plate mesophile and psychrotrophile count: 11g of the sample were added to 99 ml of peptonated water (0.1% w/v) and placed in petri dishes with PlateCount Agar and incubated at 35±2°C for 48 hours. For psychrotrophiles, they were incubated at 4±0.5°C for 5-7 days. The results were expressed as Log of Colony Forming Units/g, (Log CFU/g).
Determination of Salmonella. Twenty five g of cachama fillet were homogenized in 225 ml of buffered peptone and incubated at 35±2°C for 18 to 24 hours. 1 ml of the culture was added to one test tube with 10 ml of tetrathionate broth and to another with selenite, to which were added 2 drops of lugol and 2 drops of green coloring at 0.1% (w/v). The tubes were incubated at 35±2°C for 18 to 24 hours. The medium of the utilized selective culture was XLD agar and SS agar for the surface exhaustion method and incubation at 35±2°C for 24 hours. The results were reported as positive or negative.
Determination of sulphite reducing spores. 11 g of the sample were added to 99 ml of universal peptone (0.1 w/v) and diluted cultures of 1 ml, with preliminary thermal shock at 90°C for 5 minutes, in petri dishes with SPS agar with the depth method; and incubated at 35±2°C for 72 hours in anaerobiosis. The results were expressed as CFU/g.
Determination of total and fecal coliforms. 11 g of the sample were homogenized in 99 ml of Fluorocult LMX broth using a vortex and incubation at 35±2°C for 24 to 48 hours. The presence of the gas was determined with a Durham tube and by turbidity of the Brilla broth (Merck). The data were reported as Most Probable Number (MPN).
Sensory analysis. The sensory analysis was done with a descriptive test to judge the quality of the fillets by eight semi-trained panelists. The sensory attributes: appearance, color and aroma were evaluated in fresh fillets. The attributes: taste, texture and appearance were evaluated in cooked fillets. The scoring was based on a scale of four points, with 4 being the best and 1 the worst.
Experimental design and analysis of the data. A factorial design with two factors (time and preservation) was used to study the effect of the preservation treatments with propolis, liquid smoke and time of storage on the quality attributes of the fish fillets. A control and three levels were employed for the preservation treatments. For the factor of time, four storage periods were used (0, 8, 16 and 24 days). Three repetitions were used for each treatment. ANOVA was utilized on the results to evaluate the effect of preservation (A), time (B) and the A x B interaction on the quality attributes, using the software Statgraphics (StatisticalGraphics Corp. Rockville, USA). The difference between the mean of the values of the different treatments and the storage period was determined by the Least Significant Difference test (LSD) and the statistical difference was defined as p≤0.05.
RESULTS
In vitro microbiological analysis. Table 1 shows the inhibition halos that were obtained using the concentrations of 0.8, 1.2 and 1.6 mg/ml of EEP. It was observed that, in all cases, the substances inhibited the growth of the bacterial pathogens as compared to the control.
No significant differences (p>0.05) were found for the different concentrations (0.8, 1.2 and 1.6 mg/mL) when the diffusion technique was used to test the antagonism between the ethanol extract of propolis and Salmonella, E. coli, S. aureus and Clostridum sp. As a result, only the two lower concentrations were used.
Microbiological analysis of the fillets. Figure 1 show the results for the microbial growth of the mesophile and psychrotrophile microorganisms in the cachama fillets for each treatment during the 24 days of the sampling.
Figure 1 and table 2 show the values of the microbiological counts of the tests carried out on the cachama fillets, in accordance with NTC 1325 (13). The results demonstrate an initial microbial load represented by aerobic mesophiles and psychrophiles, and total and fecal coliforms, without the presence of Escherichia coli, Salmonella sp., Clostridium sp. or coagulase-positive Staphylococcus.
The mesophile count demonstrated continuous growth over the course of the storage time. The liquid smoke treatment and the control presented counts of 7.4 log CFU/g at 20 days of storage, which is microbiologically unacceptable. While, the propolis treatments still presented acceptable microbiological conditions at this time, with counts of 6.1 log CFU/g and 5.4 log CFU/g, using EEP concentrations of 0.8 mg/ml and 1.2 mg/ml, respectively.
The psychrotrophile analysis presented a similar condition, showing continuous growth over the time of storage. The highest counts were seen in the liquid smoke treatment and the control with values of 6.7 log CFU/g and 6.1 log CFU/g, respectively, at the end of the storage period. The EEP treatments presented values of 4.2 log CFU/g and 4.5 log CFU/g in the concentrations of 0.8 mg/ml and 1.2 mg/ml, respectively, for the same time period.
The presence of coliforms from the manipulation was reported in the treatments; however, the fecal coliforms presented a lower quantity with values under 3 NMP/g for the EEP treatments. Values over 3 NMP/g were seen in the liquid smoke treatment and the control. The aerobic mesophiles presented initial values of 3.8log CFU/g and the psychrophiles had values of 2.4 log CFU/g, increasing over the time of storage. There were significant differences (p<0.05) between the EEP treatments and the liquid smoke treatment, as well as the control, with the best microbiological conditions seen in the 1.2 mg/mL of EEP treatment. The cachama fillets treated with liquid smoke and the control exceeded the acceptable microbiological limits after 20 days of storage.
Sensory analysis. Figures 2 and 3 present the results of the sensory analysis for the cachama fillets treated with EEP for fresh fillet and cooked cachama fillets, respectively. The sensory tests were carried out every 8 days during the 24 days. The panelists graded the sensory attributes of color, aroma, and appearance in fresh cachama fillets and the attributes of taste, texture, and appearance in cooked fillets. In the analysis of the fresh cachama fillets the attribute color scored the highest for the 0.8 mg/mL EEP treatment. As for aroma, the scores were similar for all the EEP treatments, without significant differences (p>0.05). The appearance was preferred by the panelists in the 1.2mg/ml EEP treatment. The lowest values were seen in the liquid smoke treatment and the control, with samples from day 24 being rejected by the panelists in the evaluation of the three attributes.
The results of the sensory evaluation for the cooked cachama fillets that were preserved with EEP are presented in figure 3.
The sensory analysis for the taste attribute in the cachama fillets of the EEP treatments did not present significant differences (p>0.05). The obtained values decreased during the storage period, with values of 2.20 at the end, which were acceptable to the judges. The liquid smoke treatment and the control were rejected by the evaluators at the end of the storage period. The analyses of texture and appearance produced similar results for the EEP treatments, with no significant difference (p>0.05). However, the liquid smoke treatment and the control presented low values starting at day 16 and values that were rejected by the judges on day 24 of the storage.
DISCUSSION
In vitro microbiological analysis. Inhibition halos were observed with diameters starting at 8 mm for the evaluated concentrations. The diameter of the halos is considered a inhibition test, in accordance with other authors (14), the diffusion method has been used and the antimicrobial activity of EEP against S. aureus has been reported in different regions of Argentine, presenting inhibition halos with diameters starting at 9mm, which were considered as important antimicrobial activity.
It has been reported that EEP concentrations of 1.25 mg/ml have inhibition activity against Gram positive bacteria and 5 mg/ml concentrations have inhibition activity vs. Gram negative bacteria (15), values that surpass those reported in this study.
On the other hand, Palomino et al (16) indicated that bacteria such as E. coli, S. aureus and S. tiphi did not present a reduction in growth in the presence of ethanol extracts of propolis, while the Gram positive bacterium B. subtilis was sensitive to concentrations of 1.0 and 10.0 mg/ml, presenting a bacteriostatic effect at 1.0 mg/ml and a bactericide effect at 10.0 mg/ml, with 8.0mm inhibition halos. And so, the higher antimicrobial effect of propolis against Gram positive bacteria and the lower effect against Gram negative bacteria are well known. The results obtained for E. coli do not agree with those reported by Brehm-Stecher and Jonson (17), who showed that Gram negative bacteria, such as E. coli, possess an additional membrane called the “OM structure” that confers a higher degree of resistance against antimicrobial agents. Equally, Kim and Chung (18) did not report any evidence of inhibition of Salmonella Typhimurium and Escherichia coli when 2.3 mg/mL of ethanol extract of propolis was used. The results of the present study showed considerable inhibition of Salmonella spp., and E. coli, however some constituents, the application dose, the probable presence of non-volatile compounds (3), of course, the botanic origin may contribute to the variation in the antimicrobial effect of propolis on the microorganisms.
Microbiological analysis of the fillets. According to Bankova (2), a dose of EEP that would be safe for human consumption is 1.4 mg/kg of body weight/day, or approximately 70mg/day in adults. As a reference, benzoic acid and sodium benzoate were used, which are listed as food additives and cataloged as GRAS (generally recognized as safe) by the FDA. The acceptable daily intake (ADI) of both substances has values between 0 and 5 mg/kg of body weight. As a result, EEP can successfully inhibit microorganisms such as Salmonella, E. coli, S. aureus and Clostridum sp., at levels that are safe for human consumption and, as a consequence, can be used as a biopreservative or a food preservative with an unspecified antibacterial action (9).
Melliou et al (19) reported that EEP from Greece inhibited four different species of Gram negative bacterium (E. coli, E. cloacae, K. pneumoniae, P. aeruginosa). Equally, EEP from Bulgaria inhibited 90.0% of the tested Gram negative bacteria (20), while Da Silva et al (21) did not find an inhibitory activity in extracts of propolis from Brazil and Bulgaria against a strain of E. coli. Furthermore, extracts of propolis from Brazil and Korea inhibited Gram negative bacteria such as S. Typhimurium, but did not inhibit Pseudomonas aeruginosa (22). In general, it is reported that the antimicrobial spectrum is related to the presence of terpenoid compounds. Although more than 300 compounds have been identified in propolis samples, the biological activity must be principally due to some of the scarcer substances, such as flavonoids, terpenes, phenolic acid and their esters, with antimicrobial properties, and to a synergistic combination (1, 2). This could offer an explanation for the antimicrobial effectiveness for a microorganism, depending on the origin of the propolis, since the components are determinants for some bacterial strains.
Sensory analysis. The use of EEP as a preservative could influence the sensory attributes, principally affecting taste due to the presence of ethanol compounds, which, in some cases, could negatively affect the final taste of the fish. The results of the present study demonstrate that the presence of ethanol compounds did not negatively affect the perception of the evaluators, which is in agreement with other authors (23). Furthermore, the thermal shock treatment allowed for the removal of intramuscular bones without negatively affecting the sensorial acceptance, which is also in agreement with other authors (24).
In conclusions the EEP used in this study could be effective for the control of Gram positive bacteria and some Gram negative bacteria that are present in cachama fillets; and could be an alternative to the use of chemical preservatives.
REFERENCES
1. Popova M, P., Bankova V, S., Bogdanov S, Tsvetkova I, Naydenski C, Marcazzan G, Luigi, et al. Chemical characteristics of poplar type propolis of different geographic origin. Apidologie 2007;38(3):306. [ Links ]
2. Bankova V. Recent trends and important developments in propolis research. J Evid Based Complementary Altern Med 2005;2(1):29-32. [ Links ]
3. Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002;73:S1-S6. [ Links ]
4. Silici S, Kutluca S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J Ethnopharmacol 2005;99(1):69-73. [ Links ]
5. Drago L, De Vecchi E, Nicola L, Gismondo M. In vitro antimicrobial activity of a novel propolis formulation (Actichelated propolis). J Appl Microbiol 2007;103(5):1914-21. [ Links ]
6. Sawaya AC, Souza KS, Marcucci MC, Cunha I, Shimizu MT. Analysis of the composition of Brazilian propolis extracts by chromatography and evaluation of their in vitro activity against gram-positive bacteria. Braz J Microbiol 2004;35(1-2):104-9. [ Links ]
7. Erkmen O, Özcan MM. Antimicrobial effects of Turkish propolis, pollen, and laurel on spoilage and pathogenic food-related microorganisms. J Med Food 2008;11(3):587-92. [ Links ]
8. Cho J, Kim Y, Kwon M. Antibacterial effects of propolis extracts on pathogenic bacteria. J East Asian Soc Dietary Life 2005(15:4):57-64. [ Links ]
9. Tosi EA, Ré E, Ortega ME, Cazzoli AF. Food preservative based on propolis: Bacteriostatic activity of propolis polyphenols and flavonoids. Food Chem 2007;104(3):1025-9. [ Links ]
10. Mascheroni E, Guillard V, Nalin F, Mora L, Piergiovanni L. Diffusivity of propolis compounds in Polylactic acid polymer for the development of anti-microbial packaging films. J Food Eng 2010;98(3):294-301. [ Links ]
11. Kalogeropoulos N, Konteles SJ, Troullidou E, Mourtzinos I, Karathanos VT. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem 2009;116(2):452-61. [ Links ]
12. Certificación ICdNTy. Norma técnica Colombiana NTC 1443. Productos de la pesca y acuicultura. Pescado entero, medallones y trozos, refrigerados o congelados. 2009. [ Links ]
13. Certificación ICdNTy. Norma Técnica Colombiana. NTC 1325. Industrias alimentarias. Productos cárnicos procesados no enlatados. Icontec Bogotá D.C; 2008. [ Links ]
14. Chaillou LL, Nazareno MA. Bioactivity of propolis from Santiago del Estero, Argentina, related to their chemical composition. Lebenson Wiss Technol. 2009;42(8):1422-7. [ Links ]
15. Mohammadzadeh S, Shariatpanahi M, Hamedi M, Ahmadkhaniha R, Samadi N, Ostad SN. Chemical composition, oral toxicity and antimicrobial activity of Iranian propolis. Food Chem 2007;103(4):1097-103. [ Links ]
16. Palomino García LR, Martínez Galán JP, García Pajón CM, Gil González JH, Durango Restrepo DL. Caracterización fisicoquímica y actividad antimicrobiana del propóleos en el Municipio de la Unión (Antioquia, Colombia). Rev Fac Nal Agr Medellín 2010; 63(1):5373-83. [ Links ]
17. Brehm-Stecher B, Johnson E. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerodiol, farnesol, bisabolol and apritone. Antimicrob Agents Chemother 2003;47(10):3357-60. [ Links ]
18. Kim Y-H, Chung H-J. The effects of Korean propolis against foodborne pathogens and transmission electron microscopic examination. N Biotechnol 2011;28(6):713-8. [ Links ]
19. Melliou E, Stratis E, Chinou I. Volatile constituents of propolis from various regions of Greece-Antimicrobial activity. Food Chem 2007;103(2):375-80. [ Links ]
20. Boyanova L, Kolarov R, Gergova G, Mitov I. In vitro activity of Bulgarian propolis against 94 clinical isolates of anaerobic bacteria. Anaerobe 2006;12(4):173-7. [ Links ]
21. Da Silva JFM, De Souza MC, Matta SR, De Andrade MR, Vidal FVN. Correlation analysis between phenolic levels of Brazilian propolis extracts and their antimicrobial and antioxidant activities. Food Chemistry 2006; 99(3):431-5. [ Links ]
22. Choi Y, Noh D, Cho S, Suh H, Kim K, Kim J. Antioxidant and antimicrobial activities of propolis from several regions of Korea. Lebenson Wiss Technol 2006;39(7):756-61. [ Links ]
23. Haš∓cčík P, Garlík J, Elimam IOE, Kačániová M, Pochop J, Bobko M, et al. Sensory quality of poultry meat after propolis application. J Microbiol Biotechnol Food Sci 2011; 2:172-186.
24. Suárez Mahecha H, Pardo Carrasco SC, Cortés Rodríguez M. Calidad físico-química y atributos sensoriales de filetes sajados biopreservados de cachama, empacados al vacío bajo refrigeración. Rev Colom Cienc Pecu 2009: 21(3):330-339. [ Links ]