Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268
Rev.MVZ Cordoba vol.19 no.3 Córdoba Sept./Dec. 2014
ORIGINAL
Thrombocyte indices in dogs infected with Ehrlichia canis and Anaplasma phagocytophilum
Índices de trombocitos en perros infectados con Ehrlichia canis y Anaplasma phagocytophilum
Funda Özata, M.Sc, Kerem Ural, Ph.D.
1University of Adnan Menderes, Faculty of Veterinary, Department of Internal Medicine. Isikli, 09016. Aydin, Turkey.
*Correspondence: uralkerem@gmail.com
Received: January 2014; Accepted: July 2014.
ABSTRACT
Objective. In the present study alterations in trombocyte numbers and trombocyte indices were investigated in 51 dogs naturally infected with E. canis and/or A. phagocytophilum. Achieved results were compared to those of 20 healty dogs comprising control group. Materials and methods. Naturally occuring vector borne diseases were diagnosed by use of a canine point-of-care ELISA kit (Snap 4Dx, Idexx). Dogs were enrolled into 3 groups as follows; II. group involved A. phagocytophilum infected dogs (n=10), III. group (n=13) E. canis+ A. phagocytophilum co-infected, and IV. group (n=28) E. canis infected dogs. Healthy controls (n=20) were enrolled in group I. Results. Mean PLT counts were significantly decreased in II., III. and IV. groups (159.6±63.5, 142.3±44.3, 148.7±33.5, respectively) in comparison to control group (370.4±28.6) (p≤0.01). Mean PCT values in groups II., III. and IV. (0.1530±0.590, 0.1531±0.0441, 0.1450±0.314, respectively) were significantly decreased in contrast to control group (0.3695±0.0283) (p≤0.01). Between PLT and PCT values, statistically significant positive correlation (p≤0.01) (r=0.988, 0.990 and 0.981, respectively) was evident among groups II., III. and IV. Conclusions. Infected dogs showed significant alterations (p≤0.01) among mean PLT and PCT values and a positive correlation was evident between those 2 parameters (p≤0.01), whereas alterations on mean MPV and PDWc were not statistically significant. Finally it was suggested that according to the aforimentioned results, PLT and PCT values may be used as valuable parameters for diagnosis and probably for monitorization and prognosis in infected dogs with Ehrlichiosis and/or Anaplasmosis.
Key words: Anaplasma phagocytophilum, Ehrlichia canis,indices, thrombocyte (Source: CAB).
RESUMEN
Objetivo. Comparar las alteraciones en el número de trombocitos y en los índices de trombocitos en perros infectados naturalmente con E. canis y A. phagocytophilum. Materiales y métodos. Los perros fueron distribuidos en cuatro grupos: Grupo I; perros sanos (n=20), Grupo II; perros infectados con A. phagocytophilum (n=10), Grupo III; perros infectados con E. canis + A. phagocytophilum (n=13) y grupo IV; perros infectados con E. Canis (n=28). Resultados. Los recuentos de PLT se redujeron significativamente (p≤0.01) en los grupos II, III y IV (159.6±63.5, 142.3±44.3, 148.7±33.5, respectivamente) en comparación con el grupo control (370.4±28.6). La media de los valores de PCT en los grupos II, III y IV fue de 0.1530±0.590, 0.1531±0.0441 y 0.1450±0.314 respectivamente, cuyas reducciones fueron significativas (p≤0.01) en contraste con el grupo control (0.3695±0.0283). Entre los valores de PLT y el PCT la correlación fue positiva (r =0.988) y significativa (p≤0.01), 0.990 y 0.981, respectivamente, entre los grupos II, III y IV. Conclusiones. Los perros infectados mostraron alteraciones significativas (p≤0.01) entre PLT y los valores del PCT, así como una correlación positiva entre esos dos parámetros (p≤0.01), mientras que las alteraciones de MPV y PDWc no fueron significativas. Se sugiere que de acuerdo con los resultados obtenidos, los valores de PLT y el PCT se pueden utilizar como parámetros valiosos para el diagnóstico y probablemente, para el monitoreo y el pronóstico en perros infectados con la Ehrlichiosis o la Anaplasmosis.
Palabras clave: Anaplasma phagocytophilum, Ehrlichia canis, indices, trombocitos (Fuente: CAB).
INTRODUCTION
The Ehrlichial organisms Ehrlichia canis (E. canis) and Anaplasma phagocytophilum (A. phagocytophilum) are both tick-borne obligate intracellular pathogens, presenting a tropism for leukocytes (1). Infection with the latter pathogens is characterized by thrombocytopenia, fever, depression, anorexia, and other relevant signs. Dogs are involved host range of E. canis and A. phagocytophilum (1).
Previous data support that platelet count may be a reliable screening test for canine monocytic ehrlichiosis (2) and canine granulocytic ehrlichiosis (3, 4) and that the severity of thrombocytopenia may increase the reliability of diagnosis (2). Albeit the diagnostic and prognostic importance of thrombocytopenia, alterations in values for platelet indices involving plateletcrit (PCT) and mean platelet volume (MPV) in response to aforementioned pathogens are still less recognized and underestimated because of scarcity information. Therefore the aim of the present study was to evaluate platelet, besides erythrocyte counts with their indices concerning cell volume and distribution, in naturally occurring E. canis and A. phagocytophilum infections among dogs in Turkey.
MATERIALS AND METHODS
Study design and subjects. The present study was enrolled among 71 dogs (32 male/39 female; aged from 1 to 9 years) reffered to the Adnan Menderes University, Faculty of Veterinary, Department of Internal Medicine and to those of privately owned small animal clinics in located in Aydin and İzmir provinces. The animals from different breeds (8 Terrier, 7 Golden Retriever, 4 Cocker spaniel, 3 Rottweiler, 2 Turkish shepherd dog, 1 for each Doberman, German shepher dog, Jack Russel, Samoyed, Pointer, Setter, Daschund, Siberian Husky, and 39 crossbred) involved were selected to those of presenting clinical signs compatible with a suspectible Ehrlichial infection such as anorexia, weight loss, fever, generalized lymphadenopathy, splenomegalia, muscle weakness, petechiae and ecchymosis, epistaxis, limb edema and/or polyarthritis. The study protocol was approved by the institutional laboratory animals ethics committee of Adnan Menderes University HADYEK (with no: B.30.2.ADÜ.0.00.00.00 - 0.50.0.4-2010-077). Besides informed written consent was obtained from all of the dogs owners prior to enrolment of the dogs participated in study. The dogs participated in the study were selected to those of 200 dogs (with or without thrombocytopenia) by use of a canine point-of-care ELISA kit for diagnosis of naturally occuring vector borne diseases (Snap 4Dx, Idexx).
Formation of study groups. Dogs with naturally occuring Ehrlichiosis and/or Anaplasmosis (n=51) were enrolled into 3 groups as follows; II. group involved A. phagocytophilum infected dogs (n=10) (7 crossbred, 1 for each Terrier, Setter and Golden Retriever), III. group (n=13) E. canis+ A. phagocytophilum co-infected (13 crossbred), and IV. group (n=28) E. canis infected dogs (10 crossbred, 5 Terrier, 3 Golden Retriever, 2 for each Rottweiler, Turkish shepherd dog and Cocker Spaniel, 1 for each Doberman, German shepherd dog, Jack Russel Terrier and Samoyed). Healthy controls (n=20), as detected by complete physical examination and were detected to be non-infected within the test kits, were enrolled in group I. All dogs enrolled were selected to those of untreated dogs against aformentioned diseases. All dogs underwent complete physical examination and related data were recorded along within necessary labaoratory analysis. Written consent was obtained from all of the dogs owners prior to enrolment of the dogs participated in study.
Haematological and Biochemical examinations. Blood samples were withdrawn from vena cephalica antebrachii into anticoagulated (EDTA) tubes. Complete blood counts were performed on referral within Abacus Junior Vet hematology analyzer with special reference to thrombocyte indices Mean Plateled Volume (MPV), Plateletcrit (PCT) and Red Distribution Width (RDW).
Serological analysis. Each sample was tested by use of an ELISA kit (SNAP 4Dx, IDEXX Laboratories, USA) in an attempt to diagnose antigen of Dirofilaria immitis, and antibodies against Anaplasma phagocytophilum, Ehrlichia canis, and Borrellia burgdorferi according to the protocol listed in the product insert. This assay detects antibodies reacting to 2 immunodominant proteins (p30 and p30-1) of E. canis, immunodominant protein (msp2) of A. phagocytophilum and the C6 peptide for B. burgdorferi, along with a circulating carbohydrate antigen of D. immitis. The test results were recorded in an Excel spreadsheet.
Statistical analysis. Descriptive statistics were performed. Correlation coefficient among those parameters were calculated within Pearson test. Clinical signs obtained based on presence/absence such as nasal discharge, anorexia, weight loss, tick infestation, muscle weakness, dermal petechiae, epistaxis, poliarthritis and lymphadenopthy among groups were subjected to Chi square test of independence. One way analysis of variance (ANOVA) was employed for data involving hematological parameters. Thus differences for mean values among groups were detected. Moreover Tukey test was applied to determine significant differences existing among group means within variance analysis.
RESULTS
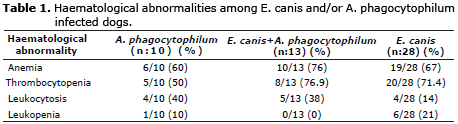
Hematological analysis. Table 1 showed hematological variables obtained. Anemia, thrombocytopenia and leukocytosis were frequently detected among dogs co-infected with E. canis and A. phagocytophilum (group III) whereas leukopenia was significantly evident in dogs infected with E. canis (group IV) in contrast to other groups. Besides only 10% of dogs infected with A. phagocytophilum presented leukopenia.
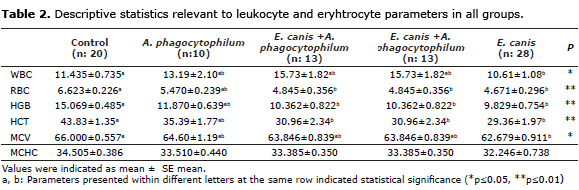
Significant differences were observed in mean WBC [between control group and IV. (E. canis) group (p≤0.05)], and RBC values [among control group and groups III. and IV. (p<0.01)] (Table 2). Variance analysis showed significant differences for mean Hb values among control group and groups III and IV (p≤0.01), and for mean Ht values among control group and groups III and IV (p≤0.01). Variance analysis showed significant differences for mean MCV values between control group and group IV (p≤0.05). Non-significant differences were detected for mean MCHC values among groups (p>0.05) (Table 2).
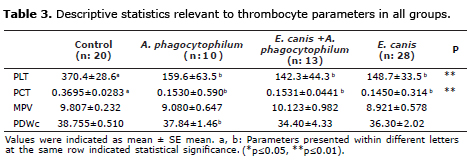
Statistically significant differences were observed for mean values of PLT and PCT for groups II, III and IV in contrast to the control group at the level of p≤0.01, respectively (Table 3). Variance analysis showed that there was no significant difference between mean MPV and PDWc values of the four groups (p>0.05).
Serological results. In vitro diagnostic rapid test kits (Snap4Dx) revealed positivity against antibodies E. canis 28/200 (14%), A. phagocytophilum 10/200 (5%) and 13/200 (6.5%) both for E. canis and A. phagocytophilum.
Clinical signs. Chi-square test was used for independence controls among groups (I, II, III, IV) and clinical findings observed based on presence (1)- or absence (0) such as nasal discharge, anorexia, weight loss, tick infestation, fever, muscle weakness, petechiae and ecchymosis, epistaxis, polyarthritis, lymphadenopathy. Nasal discharge, anorexia, weight loss, tick infestation and lymphadenopathy were dependent variables among groups at the level of p≤0.01. Other clinical signs aforementioned did not reveal statistical significance among infected groups.
Taking into account the probable transmission is through the bite of ticks for Ehrlichial organisms, active tick infestation was evident among 90%, 53.85%, and 71.43% of dogs infected with A. phagocytophilum, mixed E. canis and A. phagocytophilum and E. canis, respectively. When cases were deemed individually, a Boxer dog infected with E. canis presented distal limb edema, whereas A. phagocytophilum infected Rottweiler dog showed lameness and central nervous system signs. Dogs infected with E. canis or A. phagocytophilum showed bleeding tendency.
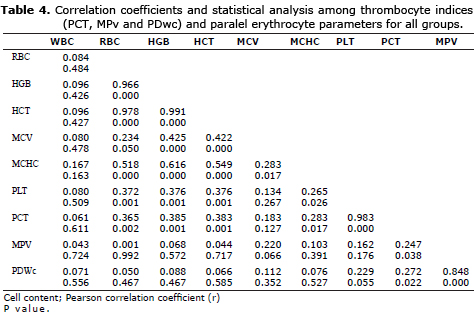
Correlation analysis for relevant hematological variables. In the present study correlation among those three thrombocyte indices(PCT, MPV and PDWc) and paralel red blood cell parameters (HCT, MCV and RDW) were investigated for all groups and for intragroup comparison. When all groups were evalauted completely Pearson correlation analysis revelaed positive strong (r:0.383) significant (p≤0.01) correlation between PCT and HCT. Besides a positive strong (r:0.372) significant (p≤0.01) correlation was also evident between PLT and RBC. Between MPV and MCV a positive but nonsignificant correlation (r=0.220; p>0.05) was found (Table 4).
Taking into account PLT and relevant thrombocyte indices, a positive strong significant correlation between PLT and PCT (r= 0.983; p≤0.01), indeed a positive but nonsignificant correlation among PLT and MPV, and PLT and PDWc (p>0.05, both) were found (Table 4).
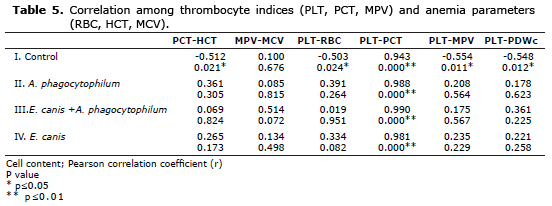
Intragroup comparison revealed a strong negative significant correlation between PCT and HCT (r:-0.512; p≤0.05) for control group. Comparative correlation among PLT and thrombocyte indices revealed a positive significant correlation between PLT and PCT (r=0.943; p≤0.01), whereas negative significant correlations among PLT and MPV (r:-0.554; p≤0.05) and PLT and PDWc (r:-0.548, p≤0.05) (Table 5).
In group II. of dogs infected with A. phagocytophilum, positive but nonsignificant (p>0.05) correlations were found among PCT and HCT (r:0.361), MPV and MCV (r:0.085), PLT and RBC (r:0.391). Within this group a positive strong (r:0.988) significant (p≤0.01) correlation was evident between PLT and PCT. Similarly in groups III. and IV. positive correlations, (r:0.990; p≤0.01) and (r:0.981; p≤0.01), respectively, were found between PLT and PCT, whereas other parameters showed non-significant correlations (Table 5).
DISCUSSION
The most commonly detected hematological alteration was thrombocytopenia, which was observed in 71.4, 90 and 76.9% of dogs infected with E. canis (IV group), A. phagocytophilum (II group) and E. canis+ A. phagocytophilum (III group). Among total population enrolled, 71.8% were infected with E. canis and/or A. phagocytophilum, and to those of 51 animals, bleeding tendency (petechia/echimoses) was determined in 13 dogs.
Thrombocytopenia is also consistent feature of A. phagocytophilum infection (5,6). Antibody-mediated haemolytic anaemia accompanying thrombocytopenia has been detected in relation to A. phagocytophilum infection in a dog (3). Indeed there is no proof of antibody-mediated immune-mediated disease. Mouse model of infection suggested that neither splenic sequestration of platelets nor antibody-mediated destruction did not have a direct effect for the acute thrombocytopenia seen with infection (5). Although haematopoietic cells including megakaryocytes were found susceptible to A. phagocytophilum, infection-induced thrombocytopenia was not suggested to be in relation with a direct effect of intracellular pathogen (7).
Taking into account all infected E. canis and/or A. phagocytophilum dogs significant differences (p≤0.01) were observed among clinical findings such as nasal discharge, appetite, tick infestation, muscle weakness, dermal petechia/echimoses, poliarthritis and lymphadenopathy in contrast to the control group. Moreover cases involved in groups II, III and IV. were dependent to the presence/absence of clinical signs aforementioned. In E. canis infected dogs (group IV.) the mean values for leukocyte responses signifricantly decreased in contrast to control group. In groups II and IV mean values for RBC, Hb and HCT were significantly decreased in contrast to control group (p≤0.01).
A common pattern of the hemopathological effects of CME may be variable during different phases of the disease (8). The effects of CME on the circulating blood cells may be accompanied by bone marrow suppression depending of the stage of the disease, thus may result with deficient production of one or more of the blood elements (8). Another research indicated that in the acute or chronic phases of CME leukopenia accompanied anemia and thrombocytopenia (9). It was suggested that a significant percentage of pancytopenic or anemic dogs with CME were serologically positive and PCR negative (9). Pancytopenia, anemia and leukopenia were already described in acute and chronic stages of CME (10). Serology positive however PCR negative dogs may be at a chronic stage, even cells are reduced due to bone marrow damage and E. canis is in the tissue, thus may not be PCR detectable. All dogs with leukocytosis were PCR positive, and 50% were also serologically positive in the latter study (9). The latter cases could be involved in acute stage of CME, as leukocytosis exist in the first 2- 3 weeks due to bone marrow hyperplasia (9). In the present study among E. canis infected dogs 14% presented leukocytosis, whereas 21% leukopenia. A. phagocytophilum infected dogs showed 40% leukocytosis, whereas 10% leukopenia. Aforementioned hematological results may be relevant to the different stages of the infection.
Automated blood cell analyzers recently are of beneficial in an attempt to provide novel data about platelets via the measurement of platelet indices, involving MPV, PDW and PCT (11, 12). Platelet indices may be of beneficial and suggests clinical data relevant to the underlying causes of thrombocytopenia in humanbeing (12), and in dogs (13, 14).
MPV has also been reported to be an indirect marker of alterations in platelet production and activity and of bone marrow response in septic patients (13-15) and inflammatory diseases in dogs (12). In a canine model of endotoxemia, elevated MPV values were reported whereas PLT and PCT decreased (16). In the latter study thrombocytopenia was significantly associated with alterations in MPV, and to those of findings may be attributable to changes in platelet production and reactivity, furthermore platelet indices may be used for diagnosis and monitoring of dogs with endotoxemia (16).
For healthy dogs Yilmaz et al (16) detected mean MPV values as 9.3 ± 0.5, whereas in a prior study min-max values of 7.9-13.5 fL (17) was detected. In 2 different studies performed among dogs infected with Babesiosis, Poland side reported MPV values of 6.1-10.1 fL in 248 dogs naturally infected with large Babesia form (18), indeed Crotian research revealed MPV min-max values of 5-13.1 fL for diseased dogs (17). For both of the studies MPV values above reference ranges may be attributable to release of immature platelets from the bone marrow due to responsive thrombopoiesis occuring in Babesisosis or beacuse of immune-mediated thrombocytopenia.
In the present study indeed expectations, mean MPV values did not present significant alterations in dogs infected with E. canis and/or A. phagocytophilum in contrast to the control group, therefore MPV may not be a sensitive platelet index in those dogs or it should be suggested that it may be an independent index at least for infected dogs involved.
The quantitation of thrombocytes in peripheral blood is well known and recognized tool. Recently, new indices related to platelet counts are provided within the use of automated hematologic analyzers (19). As platelet indices are relatively novel parameters for veterinary medicine, the scientific literature is lacking expected and expanded knowledge. The correlation between the platelet indices and parallel RBC parameters has been the subject of some studies (19).
PCT denotes the term for the percentage of blood volume occupied within the thrombocytes (20). Surfaces of cells are required for clotting alterations taking place, therefore the present authors examined PCT, as was also reported (17). In healthy dogs Yilmaz et al (16) reported PCT values of 0.33±0.01. In dogs with Babesiosis PCT values were significantly decreased before and after therapy, in contrast to the healthy dogs (17). In the latter study the reference ranges changed from 0.01 to 0.08% in dogs infected with Babesiosis, prior to therapy (17). In the present study among healthy dogs PCT was 0.3695 ± 0.0283, however the latter parameter was decrased significantly in other 3 groups with infected dogs.
In the present study mean MPV values were decreased in II and IV groups along with decrases in PCT values, indeed alterations among mean MPV and PDWc values did not show any differences compared with the control group. Furthermore thrombocytopenia was significantly correlated with the alterations in PCT. Those findings might show alterations in PLT production and reactivation or the percent of blood volume occupied within the thrombocytes, therefore we concluded that PCT, as a PLT index, may have potential value in the interpretation, diagnosis and monitorization of dogs infected with E. canis and/or A. phagocytophilum.
Taking into acoount the platelet indices, as aforementioned above PCT, MPV and PDW may be interpreted from the blood analysis (19). Albeit there is scarcity and lacking data reported in the literature regarding those parameters. MPV, actually the best recognized parameter in contrast to other relevant ones, is an index of platelet function and activation (15,). In our study mean MPV values did not show statistical differences among groups. Moreover no statistical correlation was presented between mean MPV values and paralel red blood cell parameter, MCV. In general elevated MPV should be observed in regenerative thrombocytopenia (increased peripheral loss, destruction/utilization of platelets and elevated production of platelets by marrow)(15,19). In our study as infected dogs in groups II, II and IV. did not show significant chnages among mean MPV values, it might be attributable to lacking regenerative thrombocytopenia or the duration of the diseases. The present authors did not evaluate antigenic status, solely examined antibodies against E. canis and/or A. phagocytophilum. Especially for E. canis antigenic load was not evaluated therefore active or acute stages of the disease were not determined, not allowing for entirely interpreting megakaryocytic alterations in bone marrow.
The correlation between PLT count and the three parameters and those among the three parameters and paralel RBC indices were studied. In the present study correlation among those three PLT indices (PCT, MPV and PDWc) and paralel RBC parameters (HCT, MCV and RDW) were investigated for all groups and for intragroup comparison. When all groups were evalauted completely Pearson correlation analysis revelaed positive strong (r:0.383) significant (p≤0.01) correlation between PCT and HCT. Besides a positive strong (r:0.372) significant (p≤0.01) correlation was also evident between PLT and RBC.
Taking into account PLT and relevant PLT indices, a positive strong significant correlation between PLT and PCT (r=0.983; p<0.01), intragroup comparison revealed a strong negative significant correlation between PCT and HCT (r:-0.512; p≤0.05) for control group. Comparative correlation among PLT and PLT indices revealed a positive significant correlation between PLT and PCT (r=0.943; p≤0.01), whereas negative significant correlations among PLT and MPV (r:-0.554; p≤0.05), besides PLT and PDWc (r:-0.548, p≤0.05) (Table 5). Similarly a positive significant correlation between PLT and PCT was detected in groups II, III and IV.
In conclusion, regarding to the data achieved from the present study, it may be suggested that in dogs infected with E. canis and/or A. phagocytophilum, RBC and PLT indices should be interpreted along, in an attempt to scrutinize anemia and/or trombocytopenia. Infected dogs showed significant alterations (p≤0.01) among mean PLT and PCT values and a positive correlation was evident between those 2 parameters (p≤0.01), whereas alterations on mean MPV and PDWc were not statistically significant. Finally it was suggested that according to the aforimentioned results, PLT and PCT values may be used as valuable parameters for diagnosis and probably for monitorization and prognosis in infected dogs with mentioned agents.
Acknowledgement
This study was summarized paritally from the master thesis of Funda OZATA, M.Sc., DVM, under the advisory of Dr. Kerem URAL, DVM and was funded by Adnan Menderes University Research Projects Funding Unit with project number VTF-11025.
REFERENCES
1. Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev 1991; 4:286-308. [ Links ]
2. Bulla C, Takahira RK, Araújo JP, Trinca AL, Lopes SR, Wiedmeyer CE. The relationship between the degree of thrombocytopenia and infection with Ehrlichia canis in an endemic area. Vet Res 2004; 35(1):141-146. [ Links ]
3. Bexfield NH, Villiers EJ, Herrtage ME. Immune-mediated haemolytic anaemia and thrombocytopenia associated with Anaplasma phagocytophilum in a dog. J Small Anim Pract 2005; 46:543-548. [ Links ]
4. Mazepa AW, Kidd LB, Young KM, Trepanier LA. Clinical presentation of 26 Anaplasma phagocytophilum seropositive dogs residing in an endemic area. J Am Anim Hosp Assoc 2010; 46(6):405-412. [ Links ]
5. Borjesson DL, Simon SI, Tablin F, Barthold SW. Thrombocytopenia in a mouse model of human granulocytic ehrlichiosis. J Infect Dis 2001; 184:1475-1479. [ Links ]
6. Lester SJ, Breitschwerdt EB, Collis CD, Hegarty BC. Anaplasma phagocytophilum infection (granulocytic anaplasmosis) in a dog from Vancouver Island. Can Vet J 2005; 46:825-827. [ Links ]
7. Granick JL, Reneer DV, Carlyon JA. Anaplasma phagocytophilum infects cells of the megakaryocyte lineage through sialyated ligands but fails to alter platelet production. J Med Microbiol 2008; 57:416-423. [ Links ]
8. Waner T. Hematological changes in dogs infected with Ehrlichia canis. Isr J Vet Med 2008; 63:1. [ Links ]
9. Nakaghi HCA, Machado ZR, Costa TM, Andre RM, Baldani DC. Canine ehrlichiosis: clinical, hamatological, serological and molecular aspect. Cien Rural 2008; 38(3):776-770. [ Links ]
10. Castro MB, Machado RZ, de Aguino LP, Alessi AC, Costa MT. Exprimental acute canine monocytic ehrlichiosis: clinicopathological and immunopathological findings. Vet Pathol 2004; 119(1):73-86. [ Links ]
11. Boudreaux, M.K. & Ebbe, S. Comparison of platelet number, mean platelet volume and platelet mass in five mammalian species. Comp Clin Pathol 1998; 8:16-20. [ Links ]
12. Moritz, A., Walcheck, B.K. & Weiss, D.J. Evaluation of flow cytometric and automated methods for detection of activated platelets in dogs with inflammatory disease. Am J Vet Res 2005; 66:325-329. [ Links ]
13. Sullivan PS, Manning KL, Mcdonald TP. Association of mean platelet volume and bone marrow megakaryocytopoiesis in thrombocytopenic dogs: 60 cases (1984-1993). J Am Vet Med Assoc 1995; 206:332-334. [ Links ]
14. Rafaj RB, Mrljak V, Guelfi JF. Number of thrombocytes and mean platelet volume in canine babesiosis. Rev Med Vet 2005; 156:95-98. [ Links ]
15. Bath PMW, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrin 1996; 7:157-161. [ Links ]
16. Yilmaz Z, Eralp O, Ilcol Y. Evaluation of platelet count and its association with plateletcrit, mean platelet volume and platelet size distribution width in a canine model of endotoxemia. Vet Clin Pathol 2008; 159-163. [ Links ]
17. Zvorc Z, Rafaj R B, Mrljak V. Erythrocyte and platelet indices in babesiosis of dogs. Vet Arhiv 2010; 80(2):259-267. [ Links ]
18. Zygner W, Gojska O, Rapacka G, Jaros D, Wedrychowicz H. Hematological changes during the course of canine babesiosis caused by large Babesia iin domestic dogs in Warsaw (Poland). Vet Parasitol; 2007; 145,146-15 [ Links ]
19. Wiwanitkit V. Plateletcrit, mean platelet volume, platelet distribution width: Its expected values and correlation with parallel red blood cell parameters. Clin Appl Thromb Hemost 2004; 10(2):175. 50. [ Links ]
20. Khandekar MM, Khuruana AS, Deshmukh SD, Kakrani AL, Katdare AD, Inamdar AK. Platelet volume indices in patients with coronary artery disease and acute myocardial infarction: an Indian scenario. J Clin Pathol 2006; 59:146-149. [ Links ]