Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268
Rev.MVZ Cordoba vol.19 no.3 Córdoba Sept./Dec. 2014
ORIGINAL
Transient GUS gene expression in cassava (Manihot esculenta Crantz) using Agrobacterium tumefaciens leaf infiltration
Expresión transitoria del gen GUS en yuca (Manihot esculenta Crantz) utilizando infiltración con Agrobacterium tumefaciens
Paula Díaz T,1 M.Sc, Adriana Bernal G,2 Ph.D, Camilo López C,1* Ph.D.
1Universidad Nacional de Colombia, Science Faculty, Biology Department, Manihot Biotec Investigation Group, Calle 30 N 45-03, Bogota, Colombia.
2Universidad de los Andes, Science Faculty, Biology Department, LAMFU, Cra 1 N 18A- 12, Bogota, Colombia.
*Correspondence: celopezc@unal.edu.co
Received: November 2013; Accepted: March 2014.
ABSTRACT
Objective. Assess transient gene expression of GUS in cassava (Manihot esculenta Crantz) leaves using Agrobacterium tumefaciens infiltration. Materials and methods. A. tumefaciens strains GV3101 and AGL1 containing pCAMBIA1305.2 were used to evaluate transient gene expression of β-glucuronidase (GUS). A. tumefaciens infiltration (agroinfiltration) was made using both leaves from in vitro and 1 month old greenhouse plants. Leaves were incubated in X-GLUC buffer, stained and photographed to detect GUS activity. Results. Agroinfiltration assays showed GUS transient expression in leaves of cassava varieties widely cultivated in the north coast and eastern savannah, MCOL2215 (Venezuelan) and CM6438-14 (Vergara), respectively. A. tumefaciens agressive strain AGL1 showed high efficiency inducing GUS expression in cassava leaves. Conclusions. We recommend using A. tumefaciens agressive strain AGL1 for agroinfiltration to assess transient expression in cassava leaves.
Key words: Cassava, transient gene expression, Agrobacterium tumefaciens, beta-glucuronidase (Source: NAL USDA).
RESUMEN
Objetivo. Evaluar la expresión transitoria del gen GUS en hojas de yuca (Manihot esculenta Crantz) por medio de infiltración con Agrobacterium tumefaciens. Materiales y métodos. Se utilizaron las cepas GV3101 y AGL1 de A. tumefaciens conteniendo el plásmido pCAMBIA1305.2, para evaluar la expresión transitoria del gen GUS. La infiltración de A. tumefaciens (agroinfiltración) se realizó tanto en hojas de plantas “in Vitro” como de plantas adultas de 1 mes. Las hojas se incubaron en tampón X-GLUC, se destiñeron y se fotografiaron para detectar la actividad de la enzima β-glucuronidasa (GUS). Resultados. Los ensayos de agroinfiltración en hoja muestraron la expresión transitoria del gen GUS en variedades cultivadas en la costa norte y en los llanos orientales, MCOL2215 (Venezolana) y CM6438-14 (Vergara) respectivamente, tanto en plantas “in Vitro” como en plantas adultas. La cepa hipervirulenta de A. tumefaciens AGL1 mostró una mayor eficiencia para la expresión transitoria en hojas de yuca. Conclusiones. Se recomienda utilizar la cepa AGL1 para evaluar la expresión transitoria de genes de interés por agroinfiltración en hojas de yuca.
Palabras clave: Agrobacterium tumefaciens, beta-glucuronidasa, expresión transitoria, yuca (Fuente: NAL USDA).
INTRODUCTION
Cassava (Manihot esculenta Crantz) is a perennial shrub of the Amazon (1). It was initially domesticated in the southern Amazon basin, and in the XVI century was taken to East Africa by Portuguese sailors (1). In spite of it's Amazonic origin, it is currently cultivated in more than 100 countries, principally in low-land tropical regions, with an estimated production of 240 million tons a year in 2009 (2). Cassava is considered one of the main sources of food and energy in many countries around the world and is the third source of calories in the tropics, after rice and corn (3).
The most important part of the plant for food production is the root (4). The high starch concentration (20-40% p/p) makes this crop the focus of interest as an energy source not just for human consumption but also for its industrial applications, such as bioethanol (3.5), which is currently obtained from a diversity crops including cassava.
Cassava crop is highly resistant to agroecological conditions adverse for many crops, this is mainly due to it's tolerance to drought and acidic soils (6). Additionally, a high content of cyanogenic glycosides makes it highly tolerant to generalist herbivores. All these characteristics, as well as the cassava's response to high concentrations of CO2, make this crop potentially highly resilient to global climate changes (2).
However, cassava production is compromised due to various diseases and pests that are produced by virus, fungus, bacteria and insects (4). There are currently no resistance genes to any of the diseases affecting this crop, and therefore it is important to study the molecular mechanisms (especially resistant genes) that explain immunity of the resistance varieties in order to introduce them, by means of conventional or biotechnological breeding strategies, into the susceptible cultivated varieties.
Recent advances in the development of new technologies in the area of molecular biology have produced a drastic reduction in the sequencing costs of complete genomes (7). Since the release of the Arabidopsis genome in the year 2000 (8) complete genome sequences of many plant species have been made available to the public. The complete genome sequence of cassava is currently available, which contains around 30.000 genes that codify for proteins and almost 3.500 alternative transcripts (9). The availability of this information creates a need to develop simple and efficient strategies that allow functional validation of genes.
Usually, stable genetic transformation assays have been used to express or silence genes in order to evaluate their function (10, 11). However, for the majority of plants these approximations take time and are costly, even more so for a tetraploid species with high heterozygosity and a long lifecycle, such as cassava (5). Additionally, characterizing genes using the generation of transgenic plants requires using several lines due to differences in the levels of expression between individuals and the localization of the transgene in the genome (12).
Transient expression mediated by Agrobacterium tumefaciens emerged as an alternative and quick approximation for gene characterization (13). This approximation, as well as stable transformation, is based on the use of the tumorogenic bacteria A. tumefaciens (14), which has been previously transformed with a plasmid that contains the gene of interest. Studies of the gene that codify for the β-glucuronidase enzyme (GUS) have shown that when plants are stably transformed with the gene, healthy and fertile plants are produced. The β-glucuronidase enzyme from Escherichia coli, incubated with a colorless or non-fluorescent substrate, is capable of transforming it so that it is easily visible. The commonly used substrate is X-gluc (5-bromo-4-chloro-3-indolyl glucuronide). This, together with the fact that the protein is highly stable in the cytoplasm of vegetable cells, makes it into an excellent reporter on gene expression in plants (15).
This approximation has been widely exploited in species such as Nicotiana benthamiana, N. tabacum and Arabidopsis thaliana, the first of these being a model for the study of transient expression due to easy leaf infiltration and high levels of expression over 48 hours. Additionally, this technique has been adopted in other species of agronomic and commercial interest such as beans, lettuce, pepper, and flax (10, 16).
Transient expression in leaves by infiltration of Agrobacterium tumefaciens (also known as agroinfiltration) is routinely used in several areas of investigation in molecular biology, not only to express external genes or gene silencing (11) but also to evaluate possible promoter sequences and to detect protein interactions (13). In the field of molecular plant-microbe interactions this approximation is especially used to characterize resistance genes (13, 16, 17). In cassava, in spite of the importance of transient gene expression, there are few reports on the use of this approximation, and none of them by means of leaf agroinfiltration.
The objective of this study was to transiently express GUS gene from Escherichia coli in cassava leaves by infiltration with Agrobacterium tumefaciens in order to generate a quick methodological approximation for the functional analysis of cassava genes.
MATERIALS AND METHODS
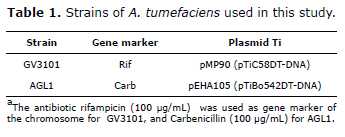
Agrobacterium tumefaciens strains. Electrocompetent cells of GV3101 and AGL1 strains of A. tumefaciens were prepared. Bacteria were grown in a YEPA medium (yeast extract 10 g/L, peptone 10 g/L, glucose 20 g/L, agar-agar 15 g/L), with their respective antibiotics (Table 1). A colony was taken to inoculate 2 mL of YEP medium (yeast extract 10 g/L, peptone 10 g/L, glucose 20 g/L). From this preculture 400 µL were taken and 300 mL were inoculated in YEP medium and incubated at 28°C to 250 rpm in darkness until the culture reached DO600 nm = 0.75 - 0.95. Washing steps were done using sterile 10% glycerol and bacteria were centrifuged at 3000 g during 10 min at 4°C. Bacteria was resuspended in sterile 1M sorbitol and stored at -80°C.
The strains were transformed by electroporation with pCambia1305.2 plasmid (Canberra, Australia), which contains the GUSPlus gene in the T-DNA interrupted by a catalase intron with the objective of evaluating the gene expression produced by the plant. The selection was performed in LB medium (tryptone 10 g/L, yeast extract 5 g/L, Sodium chloride 5 g/L), agar 15 g/L using antibiotics for each strain and kanamycin (50 µg/mL) for the plasmid selection. The colonies were picked and the presence of the GUSPlus gene was directly evaluated using PCR (Polymerase Chain Reaction). All PCR reactions were done in a final volume of 10 µL : 1x of DreamTaqTM Buffer (ThermoScientific, Waltham, MA, USA), 0.2 µM of GUSPlusFw primer (5´-CTC TTG CCA TCC TTG TCC TC-3´), 0.2 µM of GUSPlusRv primer (5´-AGC CGA AAT CTG GAA TGT TG-3´), 0.1 mM dNTPs (each), 0.25 U of DreamTaqTM DNA polymerase (ThermoScientific, Waltham, MA, USA). An initial denaturation at 95°C was done for 5 min, followed by 35 cycles of amplification (denaturation at 95°C for 30 sec, annealing at 53°C for 30 sec, extension at 72°C for 2 min), a final extension at 72°C for 10 min and a final incubation at 20°C for 10 min. In each case a sample without DNA was included as a negative control and pCAMBIA1305.2 plasmid DNA as a positive control.
Vegetal material and growth conditions. In vitro plants and one month old plants propagated from woody cuttings were used. The woody cuttings were obtained from stems of adult plants from La Vega, Cundinamarca, Colombia. All plants were grown in a greenhouse on the property of the Universidad Nacional de Colombia in Bogotá. The greenhouse temperature varied between 28 and 35°C during the day and between 22 and 27°C at night. Humidity was kept at 70% and a 12 h light/ 12 h darkness photoperiod was maintained. The plants were kept in trays with water to provide a continuous supply of moisture.
The in vitro plants were obtained from a germplasm collection at the Centro Internacional de Agricultura Tropical (CIAT), under a material transfer agreement (MTA). They were transferred to soil and kept in the greenhouse under the previously mentioned conditions for adult plants.
The 60444, CM6438-14 and MCOL2215 varieties were used. The 60444 variety has proved to be more efficient in genetic transformation in cassava (18) and is used as a model in transformation studies (19, 20). The MCOL2215 and CM6438-14 varieties are commercial ones that are widely cultivated on the Caribbean coast and the plains (Los Llanos) of Colombia (21).
Leaf agroinfiltration. An isolated colony was taken from a fresh culture for each one of the strains used (GV3101 and AGL1). It was inoculated using LB with the respective antibiotics and it was incubated at 28°C at 250 rpm in darkness until it reached DO600nm = 0.8 - 1.0. The culture centrifuged at 3000 rpm for 10 min, the supernatant was removed and it was washed using an infiltration solution of 0.5X (MES 5 mM, MgCl2 5 mM, acetosyringone 75 µM, pH 5.6). The culture was again centrifuged and was resuspended in an agroinfiltration solution 1X (MES 10 mM, MgCl2 10 mM, acetosyringone 150 µM, pH 5.6). For each culture DO600nm = 0.3 and 0.03 (depending on the experiment) was adjusted and it incubated at room temperature (20°C) for 4 hours (13).
For agroinfiltration assays young, green leaves were selected. The leaves were kept on the plant during and after agroinfiltration, and were removed just before staining to visualize the β-glucuronidase (GUS) enzyme activity. Before infiltration a small puncture was performed with a needle close to the central nerve on the underside of the leaf. The infiltration was performed using a 1 mL syringe without a needle, allowing the solution to enter through the opening made with the needle (13).
Infiltrations were done at room temperature (20°C) and the plants were kept in these conditions until the GUS staining was performed.
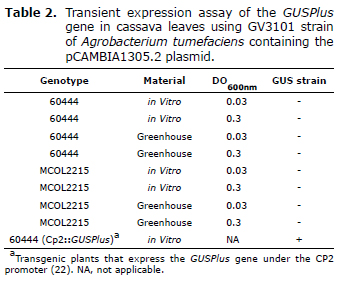
GUS staining. After three days of post-infiltration (DPI) the leaves were separated from the plant and submerged in 50 mL polypropylene tubes with a X-Gluc buffer (NaH2PO4 0.02 M, Na2HPO4 0.03 M, K3FeCN6 0.25 mM, K4FeCN6 0.25 mM, triton X-100 0.5 % (v/v), DMSO 10 % (p/v) and X-Gluc (5-bromo-4-chloro-3-indolyl β-D-glucuronide) 1 mg/mL (Sigma, St. Louis, MO, USA). In the last assays the tubes were placed in an empty chamber during 15-30 min (depending on the test) at a pressure of 20 mmHg and were incubated at 37°C for 16 h without agitation (22). In vitro cassava plants that constitutively express the GUSPlus gene were used as a positive control (22), and plants infiltrated with the infiltration solution as a negative control.
RESULTS
Use of the GV3101 strain for agroinfiltration assays in cassava plants. As a preliminary assay it was decided to evaluate in first instance transient expression only in the GV3101 strain, widely used in transient expression assays in Nicotiana benthamiana (10, 13, 23). For this, A. tumefaciens GV3101::pMP90 was transformed with the pCAMBIA1305.2 plasmid for evaluation of cassava. This strain was infiltrated in cassava leaves of the MCOL2215 and 60444 varieties, and after 3 DPI the activity of the GUS enzyme was determined by means of staining with an X-gluc buffer. As seen in Table 2, no expression of the GUSPlus gene in either of the two varieties was observed. No coloring in the in vitro plants was observed, or in the adult plants obtained from cuttings (Table 2). However, an intense blue coloring was observed in transgenic plants that express the GUSPlus gene constitutively under the CP2 promoter (22), which were used as a positive control in the GUS staining. All of these experiments were repeated twice with similar results.
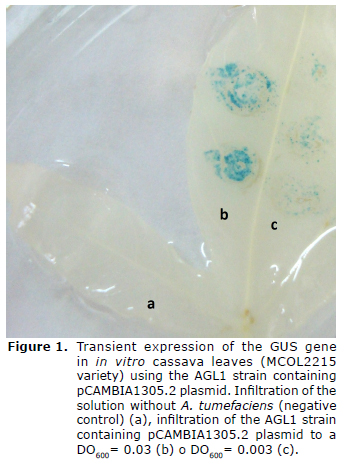
Transient expression of the GUSPlus gene in the leaves of in vitro cassava plants using the aggressive A. tumefaciens AGL1 strain. Although the GV3101 strain is frequently used in transient expression assays in a wide variety of vegetal species, the hypervirulent AGL1 strain was evaluated, which is used for the stable transformation of friable embryogenic callus (FEC) in cassava (22). To do this, in virtro plant leaves of the MCOL2215 variety were infiltrated with the AGL1 strain containing the pCAMBIA1305.2 plasmid, and after 3 DPI the activity of the GUS enzyme was determined by X-Gluc staining. In figure 1 blue-stained regions are observed at the infiltration site, which indicates biochemical activity of the GUS enzyme and therefore a transient expression of the GUSPlus gene. Additionally, the intensity of the stained region is directly related to the OD of the bacteria culture used in the infiltration (Figure 1). This same assay was done in in vitro plant leaves of the 60444 variety with similar results (data not shown). However, in spite of having used the same infiltration conditions with Agrobacterium, blue staining was not obtained in adult plants from cuttings for either of the two varieties of cassava used (data not shown).
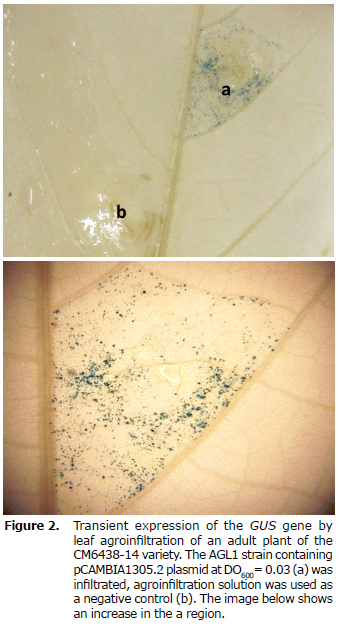
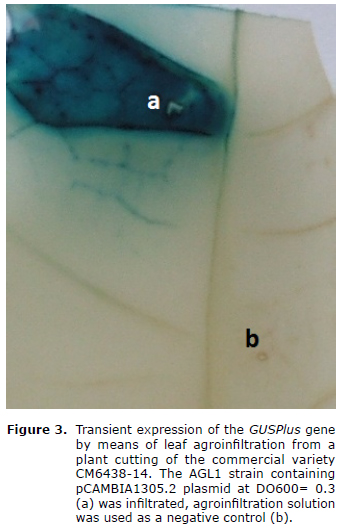
Infiltration of the X-gluc buffer in vacuum in leaves from cuttings. While cassava leaves from in vitro plants are thin and membranous, cassava leaves from plants from cuttings are thicker and have a slightly leathery consistency, and produce a white latex exudate. These characteristics of the leaves of plants from cuttings generate greater resistance to liquid penetration to the tissues. The X-Gluc buffer's inaccessibility in the infiltrated tissue could explain the negative results in detecting GUS enzyme activity when performing the infiltration of leaves from cuttings. In order to efficiently detect GUS enzyme activity, the vacuum effect on the X-Gluc buffer entering the infiltrated tissue was evaluated. The leaves of adult plants were subject to a vacuum chamber for 15 min with the purpose of promoting the entrance of the X-Gluc buffer into the tissue. In figure 2 the infiltrated region has blue spots, which demonstrates the biochemical activity of the GUS enzyme and therefore the transient expression of the GUSPlus gene in leaves from cuttings of the CM6438-14 commercial variety.
In order to obtain greater levels of transient expression in adult leaves from cuttings, incubation in the vacuum chamber was increased to 30 minutes with -20mmHg pressure. In figure 3 an intense blue stained region was observed taking up the whole infiltrated region, corresponding to the transient expression of the GUSPlus gene in leaves of cuttings from the CM6438-14 commercial variety.
DISCUSSION
Transient expression by means of Agrobacterium tumefaciens leaf infiltration is a widely used tool in several fields of plant molecular biology (10, 11, 13-17).
In cassava, protocols of stable genetic transformation have been standardized using friable embryogenic callus (FEC) from model cultivar cv. 60444 (18-20). This has allowed the introduction of characteristics of agronomic interest, which by traditional breeding may have taken much longer. Considering the long lifecycle of cassava, it has been established that generation of improved varieties can take between 4 to 7 years (4,18,20). However, in cassava production process from FEC to the generation of transgenic plants can take between 12 to 16 months (18,19). Therefore, the possibility of quickly and efficiently evaluating gene candidates to be stably transformed by means of transient expression in cassava leaves could become a useful tool previous to stable genetic transformation. This will help diminish costs and time in the stable transformation of genes that can cause adverse effects in the plants. For example, the introduction of toxicity genes will not allow plants to be regenerated. Also, the introduction of resistance genes whose constitutive expression would cause cellular death (one of the principal plant response mechanisms to pathogens) will not allow plant regeneration. On the contrary, transient expression of putative R genes in leaves followed by later inoculation with a pathogen will allow a quick validation of their role in resistance.
In this study, transient expression of the GUSPlus gene was evaluated in cassava leaves by A. tumefaciens infiltration. Although activity of the GUSPlus gene was not detected in leaves of the MCOL2215 variety and cv. 60444 when using the GV3101 strain, the possibility that this strain could also be able to promote transient expression in cassava is not dismissed, though with less efficiency compared to the hypervirulent AGL1 strain. It has been found that the efficiency of transient expression by agroinfiltration is highly dependent on the strain of A. tumefaciens and the genotype of the plant (10, 13). It is possible that particular genotypes (varieties) of plants are capable of specifically recognizing certain types of strains and cause resistance; avoiding the introduction of T-DNA of A. tumefaciens, which would be reflected in low efficiencies in the transient expression genes of reporter genes or of interest. Preliminary data in our investigation group showed that in cassava greater levels of expression of the GUSPlus gene are obtained when the AGL1 strain is used, compared with LBA4404 and GV3101 strains (data not published). This could suggest that varieties of cassava are more sensitive to this type of strain.
These results additionally show consistency and structure of the leaves dependence for the detection of transient expression of the GUSPlus gene. In vitro plants leaves are thinner with a membranous consistency, rendering them adequate for agroinfiltration assays in cassava. On the other hand, plants from cuttings have thicker leaves with a slightly leathery consistency, which combined with the production of a latex exudate, restrict the entrance of aqueous solutions to the interior of the leaf. However, as observed in figures 2 and 3, this limitation is overcome by the use of the vacuum chamber which decreases pressure temporarily. The above, followed by the re-establisement of atmospheric pressure leads to the entrance of the solution to the interior of the tissue. Optimization in assays of transient expression by A. tumefaciens diminishing pressure by using vacuum chambers has already been previously reported for several plant species (10, 24).
In summary, these results suggest the use of the hypervirulent AGL1 strain and in vitro plants to perform transient expression assays by means of agroinfiltration in cassava leaves.
Acknowledgments
Paul Chavarriaga and members of the Manihot Biotec investigation group for scientific advice in the development of assays and the enriching discussions. To the Universidad Nacional de Colombia, Vicerrectoría Académica, PDT is beneficiary of the BESP scholarship.
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
1. Olsen KM, SNPs, SSRs and inferences on cassava's origin. Plant Mol Biol 2004; 56(4):517-26. [ Links ]
2. Taylor NJ, Fauquet CM, Tohme J. Overview of Cassava Special Issue. Tropical Plant Biol 2012; 5:1-3. [ Links ]
3. FAO. Cassava's huge potential as 21st Century crop. [en línea]. Roma: Organización de las Naciones Unidades para la Agricultura y la Alimentación. 2013. [consultado 30 Mayo 2013]. URL disponible en: http://www.fao.org/news/story/en/item/176780/icode/. [ Links ]
4. Ceballos H, Okogbenin E, Pérez JC, López-Valle LAB, Debouck D. Cassava. In: Bradshaw JE, editor. Root and tuber crops. London: SpringerLink; 2010. [ Links ]
5. Balat M, Balat H. Recent trends in global production and utilization of bio-ethanol fuel. Applied Energy 2009; 86(11):2273-82. [ Links ]
6. El-Sharkawy MA. Cassava biology and physiology. Plant mol biol 2004; 56(4):481-501. [ Links ]
7. Von Bubnoff A. Next-Generation Sequencing: The Race Is On. Cell 2008; 132(5):721-3. [ Links ]
8. Yamada K, Lim J, Dale JM, Chen H, Shinn P, Palm CJ, et al. Empirical Analysis of Transcriptional Activity in the Arabidopsis Genome. Science 2003; 302(5646):842-846. [ Links ]
9. Prochnik S, Marri PR, Desany B, Rabinowicz PD, Kodira C, Mohiuddin M, et al. The Cassava Genome: Current Progress, Future Directions. Tropical Plant Biol 2012; 5:88-94. [ Links ]
10. Wroblewski T, Tomczak A, Michelmore R. Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol J 2005; 3(2):259-73. [ Links ]
11. Voinnet O, Rivas S, Mestre P, Baulcombe D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 2003; 33:949-56. [ Links ]
12. Kohli A, Twyman RM, Abranches R, Wegel E, Stoger E, Christou P. Transgene integration, organization and interaction in plants. Plant Mol Biol 2003; 52(2):247-258. [ Links ]
13. Ma L, Lukasik E, Gawehns F, Takken FLW. The Use of Agroinfiltration for Transient Expression of Plant Resistance and Fungal Effector Proteins in Nicotiana benthamiana Leaves. Cap: Bolton MD, Thomma BPHJ, editors. Plant Fungal Pathogens: Methods and Protocols. Amsterdam: SpringerLink; 2012. [ Links ]
14. McCullen CA, Binns AN. Agrobacterium tumefaciens and Plant Cell Interactions and Activities Required for Interkingdom Macromolecular Transfer. Annu Rev Cell Dev Biol 2006; 22(1):101-27. [ Links ]
15. Lee M, Yang Y. Transient Expression Assay by Agroinfiltration of Leaves. In: Salinas J, Sanchez-Serrano JJ, editors. Arabidopsis protocols. New Jersey: Humana Press Inc; 2006. [ Links ]
16. Dodds PN, Lawrence GJ, Catanzariti AM, Teh T, Wang CI, Ayliffe MA, et al. Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc Natl Acad Sci USA 2006; 103(23):8888-93. [ Links ]
17. Leckie BM, Neal Stewart C. Agroinfiltration as a technique for rapid assays for evaluating candidate insect resistance transgenes in plants. Plant cell rep 2010; 30(3):325-34. [ Links ]
18. Taylor N, Chavarriaga P, Raemakers K, Siritunga D, Zhang P. Development and application of transgenic technologies in cassava. Plant mol biol 2004; 56(4):671-88. [ Links ]
19. Bull SE, Owiti JA, Niklaus M, Beeching JR, Gruissem W, Vanderschuren H. Agrobacterium-mediated transformation of friable embryogenic calli and regeneration of transgenic cassava. Nat Protoc 2009; 4(2):1845-54. [ Links ]
20. Sayre R, Beeching JR, Cahoon EB, Egesi C, Fauquet C, Fellman J, et al. The BioCassava Plus Program: Biofortification of Cassava for Sub-Saharan Africa. Annu Rev Plant Biol 2011; 62(1):251-72. [ Links ]
21. Alzate A M, Vallejo F, Ceballos H, Pérez J C, Fregene M. Variabilidad genética de la yuca cultivada por pequeños agricultores de la región Caribe de Colombia. Acta agronómica 2010; 59(10):385-393. [ Links ]
22. Beltrán J, Prías M, Al-Babili S, Ladino Y, López D, Beyer P, et al. Expression pattern conferred by a glutamic acid-rich protein gene promoter in field-grown transgenic cassava (Manihot esculenta Crantz). Planta 2010; 231(6):1413-24. [ Links ]
23. Kim MJ, Baek K, Park C-M. Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant cell Rep 2009; 28(8):1159-67. [ Links ]
24. Simmons CW, VanderGheynst JS, Upadhyaya SK. A model of Agrobacterium tumefaciens vacuum infiltration into harvested leaf tissue and subsequent in planta transgene transient expression. Biotechnol Bioeng 2009; 102(3):965-70. [ Links ]