Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista MVZ Córdoba
versão impressa ISSN 0122-0268
Rev.MVZ Cordoba vol.20 no.1 Córdoba jan./abr. 2015
ORIGINAL
Enterotoxigenic Genes in strains of Staphylococcus spp., isolated from cheese made in Pamplona-Colombia
Genes enterotoxigénicos en cepas de Staphylococcus spp., aisladas a partir de queso elaborado en Pamplona-Colombia
Fanny Herrera A,1* Ph.D, Jesús Santos B,2 Ph.D.
1Universidad de Pamplona, Facultad de Ciencias Básicas, Departamento de Microbiología, Grupo de Investigación en Microbiología y Biotecnología (GIMBIO). Ciudad Universitaria, Pamplona, Norte de Santander, Colombia.
2Universidad de León, Facultad de Veterinaria, Departamento de Higiene y Tecnología de los Alimentos. Campus de Vegazana, A.A. Nro. 24071, León, España.
*Correspondence: fannyh@unipamplona.edu.co
Received: April 2014; Accepted: November 2014.
ABSTRACT
Objective. To determine the incidence of coagulase-positive strains of enterotoxigenic Staphylococcus in doble crema (double cream) cheese samples produced in Pamplona. Materials and methods. Bacterial isolation was performed following the routine method for coagulase positive Staphylococcus provided by the Colombian Technical Standard 4779, by using Baird Parker medium with confirmation of typical colonies by performing the coagulase test. Detection of genes for principal enterotoxins was done by PCR. Results. The prevalence of coagulase positive Staphylococcus in cheese samples was 31%, with 27% of the samples failing to meet the requirements of the NTC 750. In 24.6% of the studied isolates, genes for enterotoxin production were detected. The presence, in the isolated strains, of genes for SEB, SEA and SED was 18.5%, 4.6% and 3.0%, respectively. Conclusions. The significant presence of enterotoxigenic genes found in the isolates obtained from samples of double cream cheese made in Pamplona, suggests an important hazard to the health of consumers.
Key words: Coagulase, cheese, enterotoxins, prevalence, Staphylococcus (Source: MeSH).
RESUMEN
Objetivo. Determinar la incidencia de cepas de Staphylococcus coagulasa positivas, potencialmente enterotoxigénicas, en muestras de queso doble crema elaborado en Pamplona. Materiales y métodos. Se siguió el método tradicional de aislamiento de Staphylococcus coagulasa positivos estipulado por la Norma Técnica Colombiana 4779, empleando el medio de Baird Parker con confirmación de las colonias típicas mediante la realización de la prueba de la coagulasa. La detección de los genes para las principales enterotoxinas se realizó utilizando la técnica de PCR. Resultados. Se encontró una prevalencia de Staphylococcus coagulasa positivos en el 31% de las muestras; el 27% de las muestras incumplieron la NTC 750. En el 24.6% de las cepas estudiadas se detectaron genes para producción de enterotoxinas. La presencia, en las cepas aisladas, de genes para ESB, ESA y ESD fue de 18.5%, 4.6%, y 3.0%, respectivamente. Conclusiones. La significativa presencia de genes enterotoxigénicos encontrada en las cepas obtenidas a partir de las muestras de queso doble crema elaborado en Pamplona, sugiere un importante peligro para la salud de los consumidores.
Palabras clave: Coagulasa, enterotoxinas, prevalencia, queso, Staphylococcus (Fuente: MeSH).
INTRODUCTION
Foodborne Illnesses (FBI) are a growing problem to public health around the world, resulting in the ingestion of food contaminated with microorganisms, pluricellular organisms (helminthes), chemical and/or toxic substances. The most obvious clinical manifestations of FBIs are gastrointestinal: however, these illnesses can have chronic effects on health, including neurological, gynecological or immunological symptoms, as well as damage multiple organs, and cause cancer or death (1).
One of the most important etiologic agents of FBIs in the world is Staphylococcus aureus. The term “staphylococci” defines a group of Gram positive coco bacteria that divide on more than one plane to form three-dimensional cell bunches. Staphylococcus belongs to order I Bacillales of the Staphylococcaceae family (2); 18 species of Staphylococcus that affect food have been described, and coagulase positive ones such as S. aureus is the one that is most transmitted by food (3).
Staphylococci are normal inhabitants of the body surface of the majority of warm blooded animals, present in mucous and skin, and for this reason the most important source of these bacteria in food are nasal passages and food handlers that have wounds and/or boils on hands and arms; additionally, domestic animals such as cows can be bearers of this bacteria, since S. aureus is an important mastitis producer in milk-producing females, which contaminates cheese made from it (3).
Staphylococcus aureus produces a wide range of substances associated with virulence, among them enzymes, cytotoxins, exotoxins, exfoliative toxins and enterotoxins (4).
The main factor for virulence of Staphylococcus involved in staphylococcal food poisoning (SFP) is the production of enterotoxins (SE). S. aureus produces five typical toxins: SEA, SEB, SEC, SED and SEE, which produce emesis in primates. The enterotoxins that are best characterized are ESA and ESB; the most frequent outbreaks of ETAs are ESA and ESP (4). S. aureus enterotoxins are thermostable, and thus resist thermal treatments to which food is subjected (3).
FBI is acquired when consuming food where the microorganism has preformed one or several of its enterotoxins. Symptoms generally develop 6 hours after ingesting contaminated food, characterized by vomiting, nausea, abdominal spasms and diarrhea. It can cause death in persons whose autoimmune system is compromised. FBI is self-limiting and is not a notifiable illness, which means that many outbreaks and cases are not reported. In the United States of America (USA) it has been calculated that only from 1 to 5% of all food poisoning cases are reported; however, it is estimated that it causes 14% of the FBIs in the country (5). S. aureus is considered one of the principal agents causing FBIs in the world, and the pathogen is more frequently associated with cheese made with raw milk (6).
In the United States, it is calculated that more than 241,000 domestic cases of staphylococcal food poisoning (SFP) occur annually; keeping in mind that the hospitalization rate is 6.4% for confirmed laboratory cases, an average of 1,064 of these cases were hospitalized, resulting in 6 deaths (7). In France in 2008, S. aureus was confirmed as the second causative agent of FBIs after Salmonella and is the first suspected agent in 42.5% of FBI outbreaks.
In Latin America many types of fresh artisan cheese exist; within FBIs are SFPs. In provinces of countries in the region, such as Cuba and Argentina, S. aureus has been one of the principal bacteria implicated in FBI outbreaks in periods from 2004-2008 and 1993-2001, respectively, with artisan cheese being an important food involved in those outbreaks (8,9).
In Colombia, S. aureus is found among the principal microorganisms that are on the FBI surveillance network (10). Among published outbreaks is one that happened in the Atlantic Department in a school cafeteria from the Santo Tomás municipality and one that happened in the Honda municipality (Tolima), which affected people who participated in a community event; the causative agent in these outbreaks was S. aureus (11,12).
The objectives of this study were to detect the prevalence of positive Staphylococcus coagulasa in doble crema (double cream) cheese made in the city of Pamplona, establishing if the analyzed cheese samples did or did not comply with the allowable limits for this bacteria as established by the Colombian Technical Standard (NTC) 750, and also to determine the presence of genes that codify ESA, ESB, ESC, ESD and ESE enterotoxins in positive Staphylococcus coagulasa strains by means of the Polymerase Chain Reaction (PCR).
MATERIALS AND METHODS
Sampling. Over the course of a year, 100 samples of 200 g of doble crema (double cream) cheese made in Pamplona and sold in formally established places were collected. The samples were refrigerated and transported in the same conditions for immediate microbiological analysis.
Isolation of Staphylococcus. The NTC 4779 recommendation (13) was followed: 10 g of the sample was taken and homogenized in 90 mL of 0.1% peptonized water (Oxoid, Basingstoke, United Kingdom), making decimal dilutions up to 10-3; later 0.1 mL of dilutions 10-3 y 10-2 were piped (depending on the sample, at times 10-1 and 10-2 ó 10-3 and 10-4 dilutions were inoculated) on the previously prepared surface of the Baird Parker medium (Oxoid); the inoculated dilution was spread with the help of a Drigalsky loop until the surface was very dry, incubating at 35±2°C for 48 hours. After the incubation time, plates that contained from 20-200 black, shiny colonies were selected having white shrunken borders and surrounded by clear areas that contrasted with the opaque medium, areas with precipitated calcium and magnesium salts, which were then counted; a minimum of three colonies were used for the coagulase test.
Testing the coagulase. The number of colonies to be confirmed were transferred in the same number of tubes that contained 5 mL of Brain Heart Infusion broth (BHI, Oxoid), concluding a positive control, incubating at 35±2°C for 18-24 hours. After this time, 0.3 mL of each BHI broth was put into tubes that contained 0.3 mL of dehydrated plasma of rabbit-EDTA (bioMerieux SA, Marcy l'Etoile, France), and a tube of 0.3 mL of plasma as a negative control was also transferred, incubating at 35±2°C. For the first six hours they were observed hourly, in negative cases the reading was extended to 24 hours. Once finalized the incubation time, the results were interpreted, based on tubes that presented plasma coagulation, comparing them with positive and negative controls. The calculation of positive Staphylococcus coagulasa was done keeping in mind the total number of colonies after 48 hours of incubation and the proportion of colonies confirmed for the coagulase test, using as a correction factor the relation: positive colonies/examined colonies, reporting Colony Forming Units (CFU)/g.
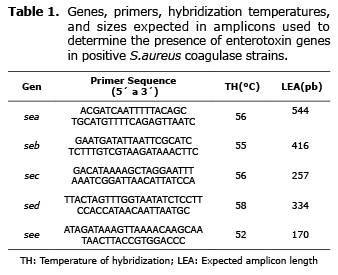
Detecting enterotoxigenic genes. From the positive coagulase strains cultivated in Soy Tripticase broth (Oxoid) and incubated at 37°C for 24 hours, 1 mL was transferred, centrifuged in a Eppendorf 5415D micro centrifuge at 12,000 rpm/3 min. Later 200 µL of Chelex (Bio-Rad, Hércules, California, USA) was added, the pellet was completely re-suspended and incubated at 56°C for 30 min. Each tube was agitated in a vortex for 10 seconds; later each tube was placed on a heating block (JP Selecta S.A, Barcelona) at 100°C for 8 minutes. Then each tube was again agitated and finally centrifuged at 12,000 rpm /3 min. The concentration of DNA used was quantified using a Spectrophotometer-UV Q3000 (Quawell, Beijing, China). Using 3 µL of the DNA (50 ng/µL approximately) the PCR was done, and for this, corresponding pairs of primers for each enterotoxin were used at a concentration of 25mM and a commercial cocktail, 5 PRIME MasterMix (Taq polimerase, buffer, dNTPs y Mg+2) (5 PRIME Hamburg, Germany). Different genes were amplified in a Mastercycler Personal (Eppendorf, Hamburg, Germany); the sequence of the primers used and the length of the amplified ones are shown in table 1 (14).
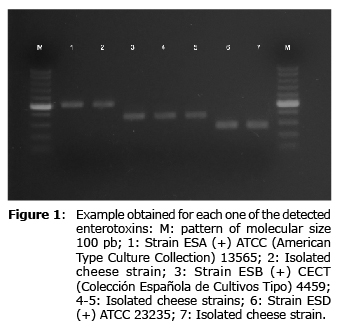
The amplification products (5 µl) were subjected to electrophoresis in agarose gel (Bio-Rad) at 1% p/v at 100 V for 1 h. Visualization was done by means of staining with RedSafeTM (iNtRon Biotechnology, INC., Sungnam, Kyungki-Do, Korea), using a transiluminator ultraviolet light, Mini-Transiluminator (Bio-Rad) and documented with the Digimage System application connected to a Canon Power Shot G11 digital camera (Major Science, Taipei, Taiwan).
RESULTS
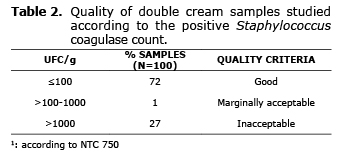
The prevalence of positive Staphylocuccus coagulase (SCP) in the analyzed samples was 31%. Additionally, 27% of the samples did not comply with NTC 750 (15), which establishes the microbiological requirements (among them positive Staphylococcus coagulase), for fresh cheese (Table 2). In 23% of the samples a concentration of SCP greater or equal to 104 UFC/g was found.
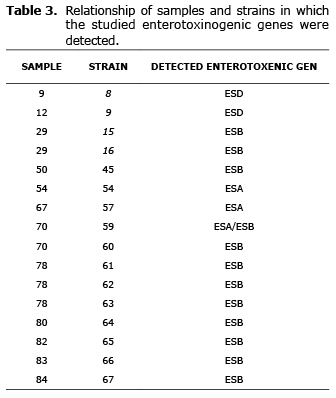
Of the 65 strains studied, genes that codified for the production of enterotoxins were found in 24.6% of the strains, which were isolated from 12 samples. The prevalence of genes for ESB, ESA and ESD in these strains was 18.5%, 4.6%, and 3.0%, respectively (Table 3, Figure 1). In one of the strains studied genes detected for ESA and ESB simultaneously. The presence of genes to produce ESC or ESE was not detected.
DISCUSSION
A high prevalence of SCP was found in the samples. These results concur with those found by Mendonça et al (16), who analyzed samples of fresh cheese sold in Brazil, determining a prevalence of 30.9%; similarly, data found by Maldonado and Llanca (17) in samples of cheese sold in Girardot, Aragua State, Venezuela, found SCP in 25% of the analyzed samples. However, these results are different from those found by other authors such as Rodríguez et al (18), who were not able to isolate SCP in samples of artisan cheese sold in Upata, Bolivar, Venezuela. Can and Celik (19) analyzed samples of cheese from Turkey, detecting SPC in 9.5% of the cheese samples. Jakobsen et al (20) detected SCP in 47.3% of samples of fresh artisan cheese made in Norway. In Colombia, Vanegas et al (10) isolated SCP in 100% of the samples sold in stores, on the street and in markets in Bogota.
On the other hand, an important percentage of samples did not comply with Colombian legislation that regulates these products (15). Different results were found by Giammanco et al, who determined that only 4% of the samples of traditional, fresh Sicilian cheese did not comply with European legislation (>103 CFU/g) (17). However, authors such as Maldonado and Llanca determined that 100% of samples of fresh cheese in the form of cakes sold in Girardot, Aragua State, Venezuela, did not comply with Covenin, the Venezuelan Commission of Industrial Regulations (>104 CFU/g). Luján et al (22) determined that 80% of the samples of fresh artisan cheese sold in three districts of Lima, Peru, did not comply with the Peruvian Technical Standards (>102 CFU/g). Komatsu et al (23) detected that 88% of the samples of cheese made in the city of Uberlândia, Brazil, did not comply with the standards established by the Ministry of Health for S. aureus (5.0 x 102 CFU/g).
The gene that codifies ESB was the main enterotoxic gene detected in this study, followed by ESA; results concur with the findings of Gücükoğlu et al (24), who analyzed samples of ice cream and found that the main enterotoxin produced was ESB. Valero-Leal (25) also found that ESB was the main enterotoxin detected in samples of milk and fresh cow cheese from Zulia State (Venezuela). On the other hand, Vanegas et al (10) detected ESA in 100% of the SCP strains isolated from artisan cheese from Bogota.
It has been determined that ESB is responsible for close to 10% of FBIs in the USA (4). Its lethal dose 50 (LD50) is 0.02mcg/kg (26), and concentrations of this toxin of just 0.4 µg/kg can induce symptoms in humans; additionally, it has been observed that individuals exposed to ESB manifest more severe symptoms than those exposed to ESA (5). On the other hand, it has been demonstrated that this enterotoxin maintains biological activity after heating to 60 °C for 16 hours, and the optimum temperature for producing it in cultures is 39.4°C (3), conditions that favor its presence in fresh cheese.
An important percentage of samples analyzed found a concentration of SCP greater or equal to 104 CFU/g; the lowest number of S. aureus cells required to produce the minimum level of enterotoxins considered necessary to provoke illness depends on the type of enterotoxin. For example, ESA has been detected in concentrations of 104 CFU/g (3); it is recognized, in general, that this value is found between 105-108 CFU/g (5). However, there is data from reported SFP outbreaks in which SCP counts are from 7.6 x 102 (27), a concentration found in 33% of the samples analyzed in this study.
Double cream cheese in Pamplona is made using raw milk and applying artisan processes where intrinsic and/or extrinsic factors do not exist that limit or control the presence of Staphylococcus aureus. Stretching, which is done between 40°C and 42°C, allows growth, since it can grow up to 47.8°C; the pH of the finished product (5.3-5.6) does not control growth or the spread of this bacteria, since it can grow in pH of up to 4.0; the percentage of salt in the cheese (3.47% NaCl), is not considered a limitation for this bacteria, since it is halotolerant and can survive in salt concentrations of up to 20% p/p (5). Additionally, contamination can occur in any stage of production, since making the product implies intense contact with handlers, utensils, environments, among others. These stages are done at temperatures above 25°C that favor the spread of the bacteria, since S. aureus has a generation time of 0.8 h at this temperature (28) and therefore produces enterotoxins.
On the other hand, the presence of this bacteria in this type of cheese could indicate contamination on the skin, mouth or nasal passages of handlers that came into direct contact with the food and did not follow minimal hygiene standards, such as the use of gloves, mouth coverings, caps or protective clothing, as well as poor cleaning of surfaces, equipment and utensils, which could be seen in some dairy microbusinesses in the province of Pamplona (29). Other sources of contamination could be milk with mastitis, work equipment and tools, air, dust and water. It is evident that inadequate cleaning and disinfecting practices increase the persistence of the bacteria in cheese processing equipment and environments, and the demonstrated resistance of S.aureus to disinfectants when a biofilm is formed is added to this (30).
In conclusion, a high prevalence of Staphylococcus was found in samples of double cream cheese made in Pamplona, reflecting inadequate hygiene practices and disinfection used all along the processing chain, so that it is necessary to train food handlers in Good Manufacturing Practices. Additionally, the storage temperature of milk and the finished product should be controlled so as to minimize the spread of the bacteria.
Finding enterotoxigenic genes in 24.6% of the analyzed strains that correspond to the toxins that are most frequently found in FBI outbreaks indicates that double cream cheese made in Pamplona can be a public health risk.
Acknowledgements
Universidad de Pamplona. Faculty of Basic Sciences, Department of Microbiology, students of the Semillero of the Investigation Group in Microbiology and Biotechnology, Paola Andrea Naranjo, Yelitza Lizcano and Vanessa Ortega.
REFERENCES
1. Haagsma J, Polinder S, Stein C, Havelaar A. Systematic review of foodborne burden of disease studies: Quality assessment of data and methodology. Int J Food Microbiol 2013; 166(1):34-47. [ Links ]
2. Yakoubou S, Xu D, Côté JC. Phylogeny of the Order Bacillales inferred from 3' 16S rDNA and 5' 16S-23S ITS nucleotide sequences. Nat Sci 2010; 2(9):990-997 [ Links ]
3. Jay J, Loessner M, Golden D. Modern Food Microbiology. Seventh Edition. New York: Springer; 2005. [ Links ]
4. Pinchuk I, Beswick E, Reyes V. Review Staphylococcal Enterotoxins. Toxins 2010; 2(8):2177-2197. [ Links ]
5. Doyle M, Buchanan R. Food Microbiology: Fundamentals and Frontiers. 4 th edition. Washington: ASM Press; 2013. [ Links ]
6. Cretenet M, Even S, Le Loir Y. Unveiling Staphylococcus aureus enterotoxin production in dairy products: a review of recent advances to face new challenges. Dairy Sci Technol 2011; 91(2):127-150. [ Links ]
7. Scallan E, Hoekstra R, Angulo F, Tauxe R, Widdowson M, Roy S, Lones J, Griffin M. Foodborne illness acquired in the United States-Major pathogens. Emerg Infect Dis 2011; 17(1):7-15. [ Links ]
8. López D, Rivero E, Martínez A, Alegret M. Enfermedades transmitidas por alimentos en Villa Clara. Rev Cubana Hig Epidemiol 2013;51(2):203-213. [ Links ]
9. Di Pietro S, Haritchabalet K, Cantoni G, Iglesias L, Mancini S, Temperoni A, Labanchi J, Barbarossa N, Garcia M, Cofre M, Rosales S, Herrero E, Bigatti R, Orellana O, Larrieu E. Vigilancia epidemiológica de enfermedades transmitidas por alimentos en la Provincia de Río Negro, Argentina, 1993-2001. Medicina 2004; 64(2):120-124. [ Links ]
10. Vanegas M, González L, Martínez A, Buitrago F. Aislamiento y caracterización de cepas de Staphylococcus enterotoxigénicos aislados de quesos en Bogotá. Rev MVZ Córdoba 2008; 13(2):1288-1293. [ Links ]
11. Guerra-Sarmiento M, Palacios-González D, Maestre-Serrano R, Baena-Del Valle J, Gómez-Camargo D. Identificación de Agentes Etiológicos Aislados de Muestras Biológicas en Brote por Intoxicación Alimentaria en el Departamento de Atlántico, Colombia 2008. Rev Cienc Biomed 2013; 4(2):233-241. [ Links ]
12. Santos M, Santos C, González J, López M. Brote de intoxicación alimentaria en el municipio de Honda, Tolima, Colombia, Junio de 2009. Inf Quinc Epidemiol Nac 2009; 14(21):321-327. [ Links ]
13. NTC 4779. Norma Técnica Colombiana. Microbiología de alimentos y alimentos para animales. Método horizontal para el recuento de Estafilococos coagulasa positiva (Staphylococcus aureus y otras especies). Bogotá, D.C.: Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC); 2007. URL Disponible en: http://tienda.icontec.org/brief/NTC4779.pdf. [ Links ]
14. Linage B, Rodríguez-Calleja J, Otero A, García-López M, Santos J. Characterization of coagulase-positive staphylococci isolated from tank and silo ewe milk. J Dairy Sci 2012; 95(4):1639-1644. [ Links ]
15. NTC 750. Norma Técnica Colombiana. Productos Lácteos. Queso. Bogotá, D.C.: Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC); 2000. URL Disponible en: http://tienda.icontec.org/brief/NTC750.pdf. [ Links ]
16. Mendonça M, Nogueira V, Keizo Y, Tassinari O, Nero L. Foodborne pathogens and microbiological characteristics of raw milk soft cheese produced and on retail sale in Brazil. Foodborne Pathog Dis 2009; 6(2):245-249. [ Links ]
17. Maldonado R, Llanca L. Estudio de la calidad del queso de mano comercializado en el municipio Girardot, estado Aragua, Venezuela. Rev Cientif FCV-LUZ 2008; 18(4):431-436. [ Links ]
18. Rodríguez C, Caldas L, Ogeerally P. Calidad sanitaria en queso artesanal tipo "telita". Upata, estado Bolívar, Venezuela. Rev Soc Venezolana Microb 2009; 29(2):98-102. [ Links ]
19. Can H, Çelik T. Detection of enterotoxigenic and antimicrobial resistant S. aureus in Turkish cheeses. Food Cont 2012; 24(1-2):100-103. [ Links ]
20. Jakobsen R, Heggeb R, Sunde E, Skjervheim M. Staphylococcus aureus and Listeria monocytogenes in Norwegian raw milk cheese production. Food Microbiol 2011; 28(3):492-6. [ Links ]
21. Giammanco G, Pepe A, Aleo A, D'Agostino V, Milone S, Mammina C. Microbiological quality of Pecorino Siciliano "primosale" cheese on retail sale in the street markets of Palermo, Italy. New Microbiol 2011; 34(2):179-185. [ Links ]
22. Luján D, Valentín M, Molina M. Evaluación de la presencia de Staphylococcus aureus en quesos frescos artesanales en tres distritos de Lima - Perú. Respyn (en línea) 2006. (Fecha de acceso 3 de Abril de 2014); 7(2). URL disponible en: http://www.respyn.uanl.mx/vii/2/articulos/quesos_frescos-1.htm. [ Links ]
23. Komatsu R, Rodrigues M, Loreno W, Santos K. Ocorrência de Staphylococcus coagulase positiva em queijo minas frescal produzido em Uberlândia-MG / Occurrence of Staphylococcus coagulase positiva in fresh minas cheese produced in Uberlândia-MG. Biosci J 2010; 26(2):316-321. [ Links ]
24. Gücükoğlu A, Çadirci Ö, Terzi G, Kevenk TO, Alişarli M. Determination of enterotoxigenic and methicillin resistant Staphylococcus aureus in ice cream. J Food Sci 2013; 78(5):738-741. [ Links ]
25. Valero-Leal K, Rivera-Salazar J, Valbuena, E, Boscán L, Valeris R, Castro G, Briñez W. Caracterización bioquímica y producción de enterotoxinas de cepas de Staphylococcus aureus aisladas de leche cruda y queso fresco artesanal en fincas del estado Zulia. Rev Cientif FCV-LUZ 2012; 22(4):303-314. [ Links ]
26. Ahanotu E, Alvelo-Ceron D, Ravita T, Gaunt E. Staphylococcal Enterotoxin B as a Biological Weapon: Recognition, Management, and Surveillance of Staphylococcal Enterotoxin. Appl Biosaf 2006; 11(3):120-126. [ Links ]
27. Kérouanton A, Hennekinne J, Letertre C, Petit L, Chesneau O, Brisabois A, De Buyser ML. Characterization of Staphylococcus aureus strains associated with food poisoning outbreaks in France. Int J Food Microbiol 2007; 115(3):369-375. [ Links ]
28. Le Marc, Valík l, Medvedová A. Modelling the effect of the starter culture on the growth of Staphylococcus aureus in milk. Int J Food Microbiol 2009; 129(3):306-311. [ Links ]
29. Daza A, Herrera F, Naranjo A. Condiciones higiénico-sanitarias aplicadas en la elaboración de queso doble crema manufacturado en tres empresas de la provincia de Pamplona-Colombia. Bistua 2013; 11(2):61-73 [ Links ]
30. Cabrera C, Gómez R, Zúñiga A. La resistencia de bacterias a antibióticos, antisépticos y desinfectantes; una manifestación de los mecanismos de supervivencia y adaptación. Colomb Med 2007;38(2):149-158. [ Links ]