Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista MVZ Córdoba
versão impressa ISSN 0122-0268
Rev.MVZ Cordoba vol.20 no.1 Córdoba jan./abr. 2015
COMUNICACIÓN BREVE
Establishing an in vitro production program for buffalo embryos (Bubalus bubalis) in Colombia
Establecimiento de un programa de producción in vitro de embriones bufalinos (Bubalus bubalis) en Colombia
Felipe Gamarra P,1 MV, Viviana Rendón V,1 MV, Aldemar Chávez R,1 MVZ, Leonardo Perez S,1 MVZ, Walter Cardona-Maya,2 Ph.D, Jesús Berdugo G,3* M.Sc.
1Genescol S.A. Carrera 17 # 58-60 Autopista Palenque-Chimita, Girón, Santander, Colombia.
2Universidad de Antioquia. Facultad de Medicina, Grupo Reproducción, Carrera 53 # 61-30, Laboratorio 534, Medellín, Colombia.
3Universidad Nacional de Colombia. Facultad de Medicina Veterinaria y de Zootecnia, Grupo de Reproducción Animal y Salud de Hato, Carrera 45 No 26-85 Edif 561B. Bogotá, Colombia.
*Correspondence: jaberdugog@unal.edu.co
Received: March 2014; Accepted: November 2014.
ABSTRACT
Objective. Evaluate the results of the standardization of the in vitro production program of buffalo embryos, using oocytes obtained by ultrasound guided oocyte puncture during the 2012 breeding season in Colombia. Materials and methods. Fifty seven buffalo females were selected for ultrasound guided transvaginal aspiration of follicles, oocytes were identified within follicular fluid, classified and transported to the laboratory and matured in vitro for 18 to 20 hours. Frozen semen of seven Mediterranean bulls were used, motile sperm was obtained using the Percoll technique and oocytes were inseminated with 2 million sperm/ml. Presumptive zygotes were cultured for 6 days, grade 1 embryos obtained were frozen using ethylene glycol. Embryos were transferred to females on day 5 during natural cycle. Results. 97 aspirations were performed on the 57 animals, in 8.2% of the aspirations no oocytes were found. 8 oocytes/aspiration were obtained. Of the 783 oocytes, 92% were classified as viable (721/783) and were fertilized. The cleavage and blastocyst rate were 23% and 19% respectively. 37 embryos were transferred and 11 pregnancies were obtained, confirmed by rectal palpation 60 days after transfer, with a pregnancy rate of 29.7%. Conclusions. The results presented here are comparable with others in literature and shows the feasibility of producing in vitro embryos and pregnancies after the standardization of current protocols, with normal and sexed semen and transfer during natural cycle in buffalo.
Key words: Buffaloes, embryo, fertilization in vitro, reproduction (Source: MeSH).
RESUMEN
Objetivo. Evaluar los resultados de la estandarización de la técnica de producción in vitro de embriones de búfalo, a partir de oocitos obtenidos por punción folicular durante la estación reproductiva del 2012 en una hacienda en Cordoba, Colombia. Materiales y métodos. Cincuenta y siete búfalas fueron seleccionadas para aspiración transvaginal de folículos guiada por ultrasonido, los oocitos fueron identificados y madurados in vitro. Se utilizó semen congelado de 7 búfalos de la raza Mediterráneo para la fertilización in vitro. La fracción móvil fue separada en un gradiente de Percoll, los oocitos fueron inseminados con 2 millones de espermatozides/mL, los presuntos cigotos fueron cultivados por 6 días y los embriones grado 1 obtenidos fueron congelados utilizando etilenglicol. Posteriormente, los embriones fueron transferidos el día 5 post estro en búfalas en ciclo natural. Resultados. En las 57 búfalas, se realizaron 97 aspiraciones foliculares, no se obtuvieron oocitos en el 8.2% de los procedimientos. Se obtuvieron en promedio 8 oocitos por búfala en cada sesión de aspiración. Se recolectaron 783 oocitos de los cuales fueron clasificados como viables el 92% (721/783), se obtuvo una taza de clivaje del 23% y de blastocistos del 19%. De 37 embriones transferidos se obtuvieron 11 preñeces, confirmadas por palpación rectal a los 60 días postransferencia, obteniéndose una taza de preñez del 29.7% Conclusiones. Los resultados presentados en este trabajo son comparables con los de la literatura, en la cual se muestra cómo es posible obtener embriones de semen convencional y sexado, además de producir gestaciones con protocolos estándar de fertilización in vitro y transferencia en ciclos con celo natural adaptados para la especie.
Palabras clave: Búfalos, embrión, fertilización in vitro, reproducción (Fuente: MeSH).
INTRODUCTION
Buffaloes are an important species due to their great adaptability in extreme environmental conditions and for their production of milk and meat. Muñoz et al (1) reported for Colombia an average milk production at 270 days of 1096±275 kg and the production of fat and protein at 270 days was 7.4 y 5.0%, respectively for lactation. For meat production, animals with increases of up to 1200 g/day are possible, which shows precocity, obtaining a final product low in fat and with a high protein content. Additionally, it has been accepted that buffaloes are more efficient than tractors in pulling conditions up to one ton and its benefits in crops have been shown, especially in palm oil (1).
Buffaloes belong to the Bovidae family, bovines, the Bubalus genus, and the species includes bubalis and carabaensis (2,3). Raising buffalo in Colombia has experienced an exponential increase in the last 15 years, and based on vaccination records, Fedegán, the Colombian Cattle Federation, reported that 160.449 heads exist in the country, females making up 70% of the population (3). Present in Colombia is the Bulbalina race, from the species Bubalus bubalis, comprised of Murrah, Mediterranean and a crossbreed of the first animals that arrived in the country, called buffalypso. From the vaccination model some technologies have been attempted to apply, especially in the area of reproduction, without satisfactory results. In Colombia, improvement programs for the species are being developed, and although reproductive biotechnologies are known and have been applied for more than 10 years, they have not spread widely to breeders (3).
The results of transferring embryos on buffalo ranches have had discouraging results, especially programs involving multiple ovulation and embryo transferring (MOET). Drost (4) reviewed the Hindu experience, including 10 years of superovulation and embryo transferring, finding 2.3 embryos per animal with a pregnancy rate of 15% and an embryo reabsorption rate of 35%.
Given the low rate of obtaining embryos by MOET, the in vitro production of embryos (PIV) became an alternative to obtaining embryos in these programs. The first buffalo embryo born in vitro was reported in India in 1990. The PIV can considerably increase the amount of embryos obtained, whether it be by slaughterhouse ovaries or live buffaloes. The average oocytes per ovary in the case of slaughterhouse is from 0.43 to 0.70 in India vs 2.4 to 3.3 in Italy (5), but if they are obtained by means of follicular aspiration guided by ultrasound (OPU), this increased up to 2.25 oocytes/ovary (6). When compared with bovines, in spite of being philogenetically similar and having similar general reproductive conditions, buffaloes have some very important differences, among them the presence of less primordial follicles and the formation of gonads (7), in consequence less follicles at birth and less amount of follicles selected in the ovulatory cycle (8).
For many years the selection system in buffalo production has had a strong maternal influence, since the offspring of the best female buffalos are the ones chosen as reproducers, and for that reason transferring embryos is chosen as the technique to be applied. Colombian breeders should increase productivity and efficiency in order to take care of market demands, especially taking advantage of the fact that animal reproduction is very efficient when it is evaluated in terms of gestation, birth rate and animal development (9,10).
With this information and the uncertainty of the results, at the end of 2008 (11) the technique of transferring embryos produced in vitro in Colombia began, with variable results. From this first experience the first animals were born in 2011 (12) which prompted an investigation group to establish an embryo production program in Colombia. The objective of this investigative study is to show the results obtained in in vitro production of buffalo embryos, report pregnancies during the 2012-2013 reproductive season, and discuss information obtained in global literature in order to apply this technology and make it a reality for the Colombian buffalo industry.
MATERIALS AND METHODS
Study site. This study took place from August 2012 and January 2013, on the Praga farm, in the Municipality Pueblo Nuevo, Córdoba, in the agroecological area of a Tropical Rain Forest (8o28'69" North 75o16'54" West), altitude 60 meters above sea level, annual rainfall 1600 mm.
Animals. 57 females, 8 buvillas and 49 multiple births with a minimum of 90 days postnatal were used, weight at 530 kg and corporal condition superior to 3.5 (scale of 1 to 5: 1 = emaciated y 5 = obese).
Follicular suction. Before aspirating the oocytes, the perineal area was washed and disinfected, and epidural anesthesia was given by means of a 4 mL injection of xylocaine. The buffalos were aspirated by means of a 20 caliber needle guided by ultrasound, connected to a convex transvaginal sound by means of an echograph (Mindray model DP 2200). The aspiration system was washed constantly with a half TCM 199 with 25 mM of hepes, supplemented with 100 USP/mL of heparin, 10% bovine fetal serum and 1% penicillin and streptomycin solution (20000 UI and 20000 µg/mL). The oocytes were aspirated by means of the union to a vacuum pump with 80 mmHg and were collected in 50 mL test tubes with 0.4 mL of heparin (Liquemine, Roche®) and identified and classified on the farm. Once identified, the oocytes were classified as A, B, C, and D (13), all the A, B and C oocytes were classified as viable and transported to the laboratory in the middle of maturation in an incubator at 37.5°C in a gassified environment, TCM 199 with 25 mM hepes, supplemented with 0.3mM cysteine, 50 µM cisteamine, 0.5 µg/ml FSH, 5 µg/mL LH and 1 ug/mL 17 β estradiol (14). All of the structures sent were subjected to maturation, no rounded structures were sent, which apparently do not have cytoplasm.
Laboratory procedure. Unless explicitly mentioned, all the reagents for the culture preparation were from Sigma-Aldrich (Sigma Chemical Co, St Louis, Mo, USA).
Once at the laboratory, the oocytes were transferred to Petri dishes to continue maturation in groups of a maximum of 20 until the moment of fertilization at 20 hours post aspiration.
For fertilization frozen conventional and sexed semen from COFA (Centro Fecundacione Artificialle, Cremona, Italy). To prepare the spermatozoids, the percoll 90-45 technique was used (15). Semen straws were thawed at 37°C for 30 seconds. Semen was placed in the upper part of the gradient, after centrifuging, the pellet was re-suspended to obtain a concentration between 1.5 to 2 million spermatozoids/ml with fertilization and TALP modified, supplementing with 0.2 mM/mL penicilamin, 0.1 mM/mL hipotaurin and 0.01 mM/mL heparin. Fertilization was done in dishes with 50 µl drops, a maximum of 20 oocytes, using specific reproducers programmed for each donator. They were cultured in the fertilization environment between 20 and 24 hours at 38.5°C, in a 5% atmosphere of CO2, 90% humidity.
Later the oocytes were denuded and the zygotes were taken to half culture, synthetic oviductal fluid (SOF) to which essential and nonessential amino acids and bovine seric albumin were added. The first environmental change was 4 days after the aspiration; due to the characteristics of the buffalo oocytes it is not possible to identify the quantity of blastomers in each structure and due to this, only the non-inseminated structures are removed. The second environmental change is done at day 6, when the first blastocysts can be observed. The formation of blastocysts begins on day 6 obtaining those that are the best quality and go to day 8, when they were frozen by means of the ethylene glycol slow freezing method (12) for direct transfer.
Some embryos were transferred to receptors in a natural cycle, day 6 post-estrum in the ipsilateral horn to the corpus luteum of this cycle's ovulation.
Statistical analysis. Data was shown with statistically descriptive values, in comparative cases the proportion comparison test was sued and a non-parametric correlation analysis was done between the variables, p>0.05 was considered statistically significant; GraphPad Prism 5 (GraphPad software, San Diego, CA) program was used.
RESULTS
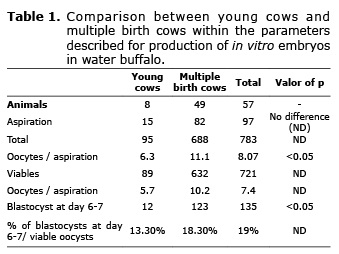
Between July 2012 and January 2013, 97 follicular aspirations were performed on 57 animals, 49 multi-birth buffalos and 8 young cows. The age of the buffalos was on average 96 months with a range from 14.5 to 206 months. The general results of the program are presented in table 1.
Of the 57 buffalos, 36 were only aspirated once. 97 aspirations were done in 8 procedures, no oocytes were obtained, the maximum of oocytes per aspiration was 35. 783 oocytes were recovered, of these 721 (92%) were classified as viable and were used to produce embryos. 8.07 total oocytes/buffalo and 7.4 oocytes/viable per aspiration. No significant differences were found in the production of oocytes/buffalo during the season, with a range from 8 to 11.
126 oocytes were fertilized with sexed semen and there were no significant differences in cleavage or in the production of embryos when compared with non-sexed semen, 20% and 17% respectively.
Of the oocytes fertilized on day 4 171 (23%) cleaved and on days 7 and 8 of the culture, 135 (19%) reached the blastocyst stage; all the embryos were frozen with ethylene glycol for direct transfer.
1.35 embryos were obtained by aspiration with a variation from 0 to 7. Of the 135 embryos obtained, 88 (65%) became blastocysts on day 6, and 47 (35%) on day 7. The production of embryos in the first aspiration in September 2012 was 10% and the last reported one in January 2013 was 20%, a significant difference (p<0.05).
In 11 female buffalos (11.3%) in which at least one oocyte was recovered no embryos were obtained, these represented 67 oocytes, 29% of these suspected zygotes did not cleave, 56% reached the state of 2 cells and 15% went from 4 to 8 cells on day 4 but stopped developing.
Additionally, buffalos that had given birth produced more oocytes and embryos than the young cows, 11.1 vs. 6.3 oocytes/buffalo and 18.3 % vs 13.3% buffalo blastocysts respectively (p<0.05). Concerning embryo development, the young cow embryos reached the blastocyst state on day 7, greater proportion than the buffalos that had given birth (56% vs 14%).
Finally 37 embryos were transferred from which 11 pregnancies were started (29%), confirmed by rectal palpation 60 days after transfer.
DISCUSSION
This study presents for the first time in world literature the use of PIV of buffalo embryos and the transfer of frozen embryos during natural heat for production of high quality buffalos.
It is seen to be a safe and reproducible procedure, complications were only seen in 1.7% of the cases; an edema could be explained as the anaphylactic reaction to the epidural anesthesia used, which was resolved spontaneously. Post aspiration complications were not present, such as adherences.
It should be kept in mind that to obtain oocytes of young animals the size of the vaginal canal was a limiting factor, since the transducer could not be used on 7 young cows (data not shown), and it is probable that in very young animals the transducer would damage the vagina canal and these animals cannot be aspirated. It was also seen that 8 buffalos could not be aspirated for anatomic reasons due to a narrow vagina or adherences of the internal organs.
The rate for obtaining oocytes in this study was 8.07 oocytes/buffalo by aspiration, which is superior to that informed in Italy 2.3 (13), Brazil 4.1-6.7 (16.17) and 1.2 in India (18). A possible explication could be the fact that the aspirations were done during the reproductive season, while some of these authors sampled their study during the whole year. The viability of this study was superior (92%) to that reported in literature (44%,45%), (16,18).
Neglia et al (19), suggest that the length of the needle and the suction system have a great influence in the quality of the oocytes found, indicating that the cells of the cumulus, poorly adhered, are detached during the aspiration process. For this study a short needle was used, that goes directly connected to the conduction system, which could explain the high viability of the oocytes obtained, even more when one of the principal parameters of classification of the oocytes are the grainy layers surrounding it.
Also, it is important to highlight that when evaluating the oocytes there are very few that can be classified as type A, since a large quantity of the oocytes do not have the appearance of a complex with adequate granular and cytoplasm; in some cases they appear as rounded structures that apparently do not have cytoplasm that would be like an atretic oocyte, which in this study weren't taken into account for the analysis. This limitation meant that almost all the oocytes obtained were used for fertilization. In conditions in the field, it is necessary to use grade A, B, C oocytes to produce embryos, and it is possible to seek ways to correctly classify oocytes of this species.
It has been reported that a greater quantity of embryos are produced when oocytes collected from live animals are used for embryo production, rather than using ovaries from a slaughterhouse (30.6±4.3% versus 18.5±1.8%). However, in literature consulted, no differences were observed in the maturation or cleavage of oocytes (18,20) when comparing these two sources of oocytes.
Pregnancy rates obtained are superior to those reported by our group in 2011 (25% vs 29%) although this difference is not significant and is in line with other reports in literature (21, 22).
Results obtained in this study using sexed semen differ from those reported by Lu et al (21), in which significant differences were observed in fertilization when sexed and conventional semen are used.
It is fundamental to understand that the production of blastocysts in vitro within a production system for in vitro embryos is not only influenced by biological factors, such as the development of follicles and the quality of oocytes, but also by factors related to laboratory procedures, the mother's nutrition and handling of the animals.
The use of frozen embryos for direct transfer facilitates establishing and managing these programs since in the properties where artificial insemination program are established, the only requirement that is needed is to change from inseminating to transferring the embryo, with time and luteal evaluation adjustments that an experienced technician can do without significantly increasing costs. From this experience, it can be said that with buffalo, biotechnological procedures that are performed with natural reproduction are successful. The pregnancy rate in this study was 29% compared with 20% (12) obtained when the receptors for transferring embryos were synchronized.
When the results of the program are analyzed from the point of view of the breeder based on the data reported here: 1.35 embryos are obtained by aspiration and a pregnancy rate of 29%, resulting in the probability of pregnancy for each aspirated buffalo of 0.39%, which means that three sessions of follicular aspiration should be done to guarantee at least one pregnancy for each animal that undergoes aspiration, calculating that to ensure at least one pregnancy 4 to 6 transferrable embryos should be produced per cycle (23). This means that for now, to see some improvement, aspiration should be done with a minimum of five donors. Investigators should strive to improve embryo production conditions and analyze donors in order to increase the possibilities of generating embryos for the improvement program.
Acknowledgements
Inversiones Colbúfalos SAS, where the procedures with animals were done.
REFERENCES
1. Cerón-Muñoz M, Gómez-Arroyave F, Ramírez-Toro J, Cifuentes T y Gutiérrez-Molina S. Parámetros genéticos para la producción de leche, grasa y proteína en búfalos de Colombia. Livestock Research for Rural Development. 2012 24(2), Article #30 URL Disponible en: http://www.lrrd.org/lrrd24/2/cero24030.htm. [ Links ]
2. Perera B. Reproductive cycles of buffalo. Anim Reprod Sci 2011; 124(3):194-9. [ Links ]
3. Berdugo Gutiérrez J. Historia de la aplicación de biotecnologías reproductivas en la cría del búfalo en Colombia. Uni-pluri/versidad 2013;12(3):87-91. URL Disponible en: http://aprendeenlinea.udea.edu.co/revistas/index.php/unip/article/viewFile/15160/13204. [ Links ]
4. Drost M. Advanced reproductive technology in the water buffalo. Theriogenology 2007;68(3):450-3. [ Links ]
5. Boni R, Roviello S, Gasparrini B, Langella M, Zicarelli L. In vitro production of buffalo embryos in chemically defined medium. Buffalo J 1999;15:115-20. [ Links ]
6. Galli C, Duchi R, Crotti G, Lazzari G. Embryo production by ovum pick up in water buffalo. Theriogenology 1998; 49(1):400. [ Links ]
7. Kumar A, Solanki V, Jindal S, Tripathi V, Jain G. Oocyte retrieval and histological studies of follicular population in buffalo ovaries. Anim Reprod Sci 1997; 47(3):189-95. [ Links ]
8. Baruselli P, Mucciolo R, Visintin J, Viana W, Arruda R, Madureira E, et al. Ovarian follicular dynamics during the estrous cycle in buffalo (Bubalus bubalis). Theriogenology 1997; 47(8):1531-47. [ Links ]
9. Aspilcueta-Borquis RR, Sesana RC, Berrocal MHM, Seno LdO, Bignardi AB, El Faro L, et al. Genetic parameters for milk, fat and protein yields in Murrah buffaloes (Bubalus bubalis Artiodactyla, Bovidae). Genet Mol Biol 2010; 33(1):71-7. [ Links ]
10. Galeazzi P, Mercadante M, Silva J, de Albuquerque L, de Camargo G, Tonhati H. Analysis of culling probability in dairy buffalo using survival models. Animal. 2010; 4(8):1325-9. [ Links ]
11. Hernández C, Castañeda S, Lopez C, Arias C, Watanabe Y, Berdugo J. Aspiración folicular guiada por ultrasonido (OPU) y producción de embriones in vitro en búfalas de agua (Bubalus bubalis) en el trópico bajo colombiano. Rev Colomb Cienc Pecu 2009; 22(3):567. [ Links ]
12. Galli C, Berdugo J, Pacheco L, Angel A, Posada I, Lazzari G, et al. First pregnancies obtained after transfer frozen-thawed buffaline embryos produced in Vitro in Colombia. Reprod Fertil Dev 2011; 24:190. [ Links ]
13. Di Francesco S, Novoa MVS, Vecchio D, Neglia G, Boccia L, Campanile G, et al. Ovum pick-up and in vitro embryo production (OPU-IVEP) in Mediterranean Italian buffalo performed in different seasons. Theriogenology 2012; 77(1):148-54. [ Links ]
14. Sharma GT, Dubey PK, Nath A, Saikumar G. Co-culture of buffalo (Bubalus bubalis) preantral follicles with antral follicles: a comparative study of developmental competence of oocytes derived from in vivo developed and in vitro cultured antral follicles. Zygote. 2013; 21(3):286-94. [ Links ]
15. Parrish JJ, Susko-Parrish JL, Leibfried-Rutledge ML, Critser ES, Eyestone WH, First NL. Bovine in vitro fertilization with frozen-thawed semen. Theriogenology 1986; 25:591-600. [ Links ]
16. Mondadori RG, Santin TR, Fidelis AAG, da Silva JS, Rumpf R, Báo SN. Ultrastructure of in vitro oocyte maturation in buffalo (Bubalus bubalis). Zygote 2010; 18(4):309-14. [ Links ]
17. Sá Filho M, Carvalho N, Gimenes L, Torres-Júnior J, Nasser L, Tonhati H, et al. Effect of recombinant bovine somatotropin (bST) on follicular population and on in vitro buffalo embryo production. Anim Reprod Sci 2009; 113(1):51-9. [ Links ]
18. Manjunatha B, Ravindra J, Gupta P, Devaraj M, Nandi S. Oocyte recovery by ovum pick up and embryo production in river buffaloes (Bubalus bubalis). Reprod Domest Animal 2008; 43(4):477-80. [ Links ]
19. Neglia G, Gasparrini B, Caracciolo di Brienza V, Di Palo R, Campanile G, Antonio Presicce G, et al. Bovine and buffalo in vitro embryo production using oocytes derived from abattoir ovaries or collected by transvaginal follicle aspiration. Theriogenology 2003; 59(5):1123-30. [ Links ]
20. Gasparrini B, Sayoud H, Neglia G, Matos DGd, Donnay I, Zicarelli L. Glutathione synthesis during in vitro maturation of buffalo (Bubalus bubalis) oocytes: effects of cysteamine on embryo development. Theriogenology 2003; 60(5):943-52. [ Links ]
21. Lu Y, Liang X, Zhang M, Wang W, Kitiyanant Y, Lu S, et al. Birth of twins after in vitro fertilization with flow-cytometric sorted buffalo (S) sperm. Anim Reprod Sci 2007; 100(1):192-6. [ Links ]
22. Galli C, Duchi R, Colleoni S, Lagutina I, Lazzari G. Ovum pick up, intracytoplasmic sperm injection and somatic cell nuclear transfer in cattle, buffalo and horses: from the research laboratory to clinical practice. Theriogenology. 2014;81(1):138-51. [ Links ]
23. Bó GA, Baruselli PS, Chesta PM, Martins CM. The timing of ovulation and insemination schedules in superstimulated cattle. Theriogenology 2006; 65(1):89-101. [ Links ]