Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista MVZ Córdoba
versão impressa ISSN 0122-0268
Rev.MVZ Cordoba vol.20 no.1 Córdoba jan./abr. 2015
CASO CLÍNICO
Terminal leptospirosis in a woolly monkey (Lagothrix lagothricha) in Colombia
Leptospirosis terminal en un mono lanudo (Lagothrix lagothricha) en Colombia
Viviana González A,1* M.Sc, Juliana Peña S,2 MV, Javier Bustamante R,1 M.Sc, Miryam Astudillo H,1 M.Sc.
1Universidad del Valle, Facultad de Salud, Grupo de Biotecnologia y Enfermedades Bacterianas, Av 1N No. 3N-03, Cali, Colombia.
2Fundacion Zoologica de Cali, Carrera 2 Oeste Calle 14, Cali, Colombia.
*Correspondence: vivianarjbr@gmail.com
Recibido: March 2014; Aceptado: November 2014.
ABSTRACT
A captive woolly monkey (Lagothrix lagothricha) displayed severe lethargy, vomiting and diarrhea. Laboratory tests revealed anemia, leukopenia, hypoproteinemia, severe azotemia and positive Leptospira IgM ELISA. The monkey was humanely euthanized and the necropsy revealed a multifocal tubulointerstitial glomerulonephritis; in addition to splenic lymphoid depletion, and interstitial pneumonia, all of which are compatible with leptospirosis. Rodent control and biosecurity measures should be done at all zoological collections in order to prevent transmission to facility personnel and to threatened mammals maintained in captivity.
Key words: Epidemiology, leptospira, primate, zoo (Source: DeCS).
RESUMEN
Se presenta el caso clínico de un mono lanudo (Lagothrix lagothricha) en cautiverio, con letargia, vómito y diarrea. Las pruebas de laboratorio revelaron anemia, leucopenia, hipoproteinemia, azotemia severa y ELISA IgM positiva a Leptospira. El mono fue humanamente sacrificado y la necropsia reveló una glomerunonefritis tubulointersticial multifocal; además de depleción linfoidea esplénica, y neumonía intersticial, los cuales son compatibles con leptospirosis. Todas las colecciones zoológicas deberían realizar protocolos de desratización y aplicar medidas de bioseguridad para prevenir la transmisión al personal y mamíferos amenazados mantenidos en cautiverio.
Palabras clave: Epidemiologia, leptospira, primate, zoológico (Fuente: DeCS).
INTRODUCTION
Leptospirosis is the most widespread rat-borne zoonosis in tropical regions, also affecting mammals other than the maintenance host in tropical regions (1,2), caused by pathogenic serovars of the spirochete Leptospira spp. Transmission occurs through direct or indirect routes with contaminated bodily fluids from domestic maintenance hosts such as Rattus spp. and Mus spp. (2). Fatalities typically arise due to renal-vascular dysfunction that leads to renal, cardiac, or respiratory failure (3). Naturally acquired infection with Leptospira is uncommon in non-human primates (4), with only scant reports of seroreactivity in wild primates (5) and asymptomatic seroconversion occurring in primates in zoos (6,7). However, captivity-associated confinement of non-native species may increase exposure to peridomestic reservoirs, promoting pathogen transmission potential to animals and personnel (6). This report details a clinical case of leptospirosis in a captive woolly monkey (Lagothrix lagothricha).
CASE REPORT
A zoo-born, adult 8 kg male woolly monkey (Lagothrix lagotricha) had been part of the collection of the Zoological Foundation of Cali, Colombia, since 1997. The monkey was a dominant male that shared the enclosure with other members of the same species. In June 2010, the monkey was reported to be severely lethargic, vomiting and passing diarrhea. On day one, anesthesia was performed in order to conduct a physical examination, and to collect blood for a complete blood count, serum biochemistry, serology, and urine for a dark-field examination and urinalysis. Antibiotic and supportive treatment was also administered under anesthesia. The physical exam determined a good body condition (4/5), dehydration of 10%, and lymphadenopathy of the submandibular, axillary, inguinal and popliteal lymph nodes.
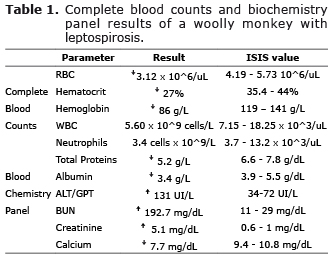
The monkey had moderate anemia with unremarkable values of both mean corpuscular volume and mean corpuscular hemoglobin, indicating a normocytic normochromic anemia characterized by severe poikilocytosis and polychromasia, with many echinocytes, and moderate Howell-Jolly bodies, in addition to leukopenia with moderate neutropenia. Platelet count was within normal International Species Information System (ISIS) ranges and no bands were detected. Of interest, the biochemical panel revealed hypoalbuminemia and hypocalcemia. We also detected marked increment of alanine transaminases and severe increase of creatinine and BUN (Table 1).
On day two, blood was drawn once more to monitor kidney function and as a treatment follow-up. There was a marked increase of creatinine (813.28 µMol/L), and electrolyte imbalance (plasma sodium 150.1 mMol/L and phosphate 4.58 mMol/L). Urinalysis revealed proteinuria, hematuria, and leukosuria (100 mg/dL and 17-20 RBC and 8-10 leucocytes per 40X field, respectively) with low bacteria counts.
The presence of detectable antibodies against Leptospira was confirmed through ELISA IgM test (12 units). According to the manufacturer, results >11 units are considered positive for anti-Leptospira IgM. These antibodies are produced during the acute phase of the disease, but may remain detectable for longer periods (2). However, diagnosis was reached considering a positive ELISA result in combination with clinical presentation and epizootiology. Urine dark field microscopy was negative for the presence of spirochetes. Due to unresponsiveness to treatment and clinical deterioration, the monkey was euthanized. In Colombia, euthanasia is performed by qualified veterinarians by administering intravenously 1 mL of a solution of sodium penthobarbital and sodium difenilhidantoine per 5 kg total body weight. The first compound acts as a non-selective central nervous system depressant and the latter is an anti-convulsant. This procedure offers a calm, rapid and humane death.
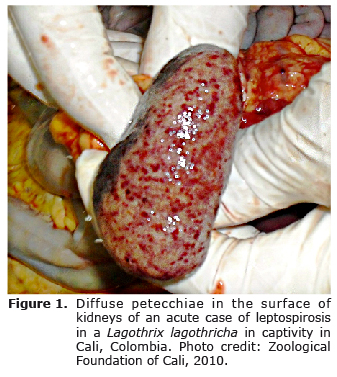
Necropsy revealed presence of abundant abdominal adipose tissue, in addition to free blood in thorax and abdomen. The kidneys (Figure 1) and lungs contained diffuse pinpoint hemorrhages; the latter also had atelectic foci and the trachea was edematous. No gross lesions were found in the spleen, liver, intestines and pancreas. The cut surfaces of the kidneys bulged and both kidneys were slightly enlarged. H&E histopathology revealed multifocal tubule-interstitial glomerulonephritis, changes in spleen associated with lymphoid depletion, and moderate interstitial pneumonia. The bloody liquid found in thorax and abdomen contained mainly bilirubin, blood cells and proteins.
DISCUSSION
This clinical case presents the first documented clinical case of leptospirosis in a captive woolly monkey. Very few confirmed cases of leptospirosis have been reported in non-human primates in the world (3). The only reported outbreak in Colombia happened in a rehabilitation facility in Cebus capucinus, C. apella and C. albifrons, where most of the primates presented diffuse jaundice and pulmonary hemorrhage (8).
This anicteric infection was characterized by leukocytopenia. The septicemic phase of leptospirosis is associated with damage to vascular lining cells, which combined with the release of bacterial toxins and inflammatory responses to the bacteria (4), account for the pathological features found in this case, including nephritis and petecchiae (2,8). This phase may be followed by a systemic inflammatory response syndrome, which may reflect a leukopenia or leukocytosis, depending on the response of each individual (4), although leukocytosis is more common (2). RBC showed severe poikilocytosis predominantly marked by echinocytes, revealing a toxic disease process with alteration of the RBC membrane. Howell-Jolly bodies showing signs of hemolytic anemia or an affected spleen; also polycromasia and microcytes, which occur in disseminated intravascular coagulation (DIC). DIC and leukopenia are common findings amongst patients with sepsis, and leptospirosis is considered a frequent cause of sepsis in tropical regions (9). Barbiturate euthanasia solutions can cause vascular damage and necrosis in different organs. However, the incidence of these artifacts is minimal or absent when recommended doses are used (10).
Serum biochemistry analysis revealed hypoproteinemia, hypocalcemia, azotemia and hypoalbuminemia, which are all indicators of a nephropathy, more specifically, acute renal failure. Although jaundice is a common clinical sign associated with leptospirosis, anicteric leptospirosis is also reported as part of the acute febrile syndrome (11). Alanine amino transferase is almost exclusively found in the primate liver; however, increased levels in the absence of jaundice can be the result of a hemolytic crisis, because it is also found in the RBC cytosol. In addition, anicteric disease can occur in peracute processes with severe liver damage and increased enzyme activity (3). The exudate collected from thorax and abdomen was most likely linked to the severe acute multi-organic inflammatory reaction.
The absence of leptospires in the urine viewed by dark-field microscopy may be a reflection of intermittent shedding, known to occur in some species. It could also be due to the bactericidal treatment administered (12).
Very few confirmed cases of leptospirosis have been reported in non-human primates in the world (3). The only reported outbreak in Colombia happened in a rehabilitation facility, where most of the primates presented diffuse jaundice and pulmonary hemorrhage (8). We confirmed leptospirosis with ELISA IgM; these antibodies may remain detectable for several months (3). However, a high IgM titer in a single serum specimen is consistent with a current or recent infection. The specific serovar involved could not be determined due to the known cross-reactivity with in test.
The fact that a single animal was infected in the enclosure might be due to individual infection susceptibility possibly related to endocrine responses (stress) from multiple sources. Behavioral modifications due to captive conditions might also influence this pattern since these folivorous animals have been seen hunting rodents inside the enclosure (unpublished data).
Although leptospirosis is not a reportable disease in Colombia, it is listed internationally as an important occupational disease in captive facilities (6), and it holds economic relevancy. Since leptospirosis diagnosis in non-human primates tends to be characterized by less-evident clinical signs and lesions, and by short-lived antibody responses (3), this clinical case provides evidence that it is important to consider it part of rule-outs or differential diagnosis in future clinical events within and outside of Colombia. Rodent control and biosecurity measures are recommended for all institutions keeping mammals in order to avoid pathogen transmission to personnel and between enclosures.
Acknowledgements
We thank Dr. Carlos Lopez DVM for his advice on clinical pathology, Dr. Sonia M. Hernandez DVM PhD for her comments, Leonor Oviedo for laboratory assistance and the staff at the Colombian Agricultural Institute (ICA) for sample provision, and to the personnel at the Zoological Foundation of Cali who were actively involved in this case.
Conflict of interests: Authors declare having no conflict of interest.
REFERENCES
1. Astudillo-Hernández M, González-Rodríguez A, Batista-Santiesteban N, Mirabal-Sosa M, Menéndez-Hernández J. Estudio seroepidemiológico de la leptospirosis humana en el departamento del Valle del Cauca, Colombia. Rev Cub Med Trop 2009; 61:152-159. [ Links ]
2. Adler B, de la Pena-Moctezuma A. Leptospira and leptospirosis. Vet Microbiol 2010; 140:287-296. [ Links ]
3. Ubb KVF, Kennedy PC, Palmer N. Pathology of Domestic Animals. San Diego: Academic Press; 2007. Pg: 503-510. [ Links ]
4. Minette HP. Leptospirosis in primates other than man. Am J Trop Med Hyg 1966; 15:190-198. [ Links ]
5. Thayaparan S, Robertson I, Amraan F, Su'ut L, Abdullah MT. Serological prevalence of Leptospiral infection in wildlife in Sarawak, Malaysia. Borneo J Res Sci Tech 2013; 2:79-82. [ Links ]
6. Forsyth MB, Morris AJ, Sinclair DA, Pritchard CP. Investigation of zoonotic infections among Auckland Zoo staff: 1991-2010. Zoonoses Public Health 2012; 59:561-567. [ Links ]
7. González-Astudillo V, Wehdeking-Hernández D, Peña-Stadlin JE, Arias-Bernal L, Lombo-Rodriguez DA, Astudillo-Hernandez M. Comparative seroprevalence of Leptospira interrogans in Colombian mammals along a climatic gradient. J Zoo Wildl Med 2012; 3:768-775. [ Links ]
8. Szonyi B, Agudelo-Florez P, Ramírez M, Moreno N, Ko AI. An outbreak of severe leptospirosis in capuchin (Cebus) monkeys. Vet J 2010; 188: 237-239. [ Links ]
9. Chierakul W, Tientadakul P, Suputtamongkol Y, Wuthiekanun V, Phimda K, Limpaiboon R, et al. Activation of the coagulation cascade in patients with leptospirosis. Clin Infect Dis 2008; 46:254-260. [ Links ]
10. Grieves JL, Dick EJ Jr, Schlabritz-Loutsevich NE, Butler SD, Leland MM, Price SE, et al. Barbiturate euthanasia solution-induced tissue artifact in nonhuman primates. J Med Primatol 2008; 37:154-161. [ Links ]
11. Assimakopoulos SF, Fligou F, Marangos M, Psilopanagioti A, Filos KS. Anicteric leptospirosis-associated severe pulmonary hemorrhagic syndrome: a case series study. Am J Med Sci 2012; 344:326-329. [ Links ]
12. Cortese VS, Behan S, Galvin JE, Penka DR, Ramsey D, Bryson WL, et al. Evaluation of two antimicrobial therapies in the treatment of Leptospira borgpetersenii serovar hardjo infection in experimentally infected cattle. Vet Ther 2007; 8:201-208. [ Links ]