Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268
Rev.MVZ Cordoba vol.20 no.3 Córdoba Sept./Dec. 2015
ORIGINAL
Effects of Kikuyu grass (Pennisetum clandestinum) age and different forage: concentrate ratios on methanogenesis
Efecto de la edad del pasto kikuyo (Pennisetum clandestinum) y la relación forraje: concentrado sobre la metanogénesis
John Ramírez,1 M.Sc, Sandra Posada O,1* Ph.D, Ricardo Noguera,1 Ph.D.
1Universidad de Antioquia, Facultad de Ciencias Agrarias, Grupo de Investigación en Ciencias Agrarias-GRICA, AA 1226, Medellín, Colombia.
*Correspondence: slposada@gmail.com
Received: September 2014; Accepted: February 2015.
ABSTRACT
Objective. To evaluate the effect of Kikuyu grass (Pennisetum clandestinum) harvested at two different ages and three forage: concentrate supplement ratios (F/C) on methane (CH4) production, dry matter digestibility (DMD), and fermentation profile using the in vitro gas production technique. Materials and methods. six treatments, resulting from the combination of pasture age (30 or 60 days) and F/C (100/0, 75/25, or 50/50) were evaluated using a 2x3 factorial design. The response variables were measured 6, 12, 24 and 48 hours after incubation. A repeated-measure over time design was used to analyze the data, and differences between means were determined with the LSMEANS procedure of SAS. Results. the youngest grass (30 days) was more digestible, produced less CH4 per gram of digestible dry matter (dDM) and more total volatile fatty acids (VFA) compared to the oldest grass (60 days; p <0.05). Reductions of the F/C ratio increased DMD and CH4 production per gram of dDM (p<0.05) but had no significant effect on VFA concentration (p>0.05). Conclusions. under in vitro conditions and pH close to neutrality, the older grass reduces DMD and increases CH4 production per gram of dDM, while a F/C reduction increases DMD and CH4 production per gram of dDM, which differs with reports conducted in vivo.
Key words: Carbohydrates, in vitro digestibility, grass maturity, methane production (Sources: CAB, USDA).
RESUMEN
Objetivo. Evaluar el efecto de la edad del pasto kikuyo (Pennisetum clandestinum) y de la relación forraje/suplemento (F/S) sobre la producción de metano (CH4), la digestibilidad de la materia seca (DMS) y el perfil de fermentación mediante la técnica in vitro de producción de gas. Materiales y métodos. Seis tratamientos, resultantes de la combinación de los factores edad del pasto (30 y 60 días) y relación F/S (100/0, 75/25 y 50/50), fueron evaluados bajo un diseño factorial 2x3. Las variables respuesta se midieron a las 6, 12, 24 y 48 horas de incubación. Para el análisis de los datos se empleó un diseño de medidas repetidas en el tiempo y las diferencias entre medias se determinaron con el procedimiento LSMEANS de SAS. Resultados. El pasto de menor edad (30 días) fue más digestible, produjo menos CH4 por gramo de materia seca digestible (MSd) y más ácidos grasos volátiles totales (AGV) que el pasto de mayor edad (60 días, p<0.05). La reducción en la relación F/S aumentó la DMS y la producción de CH4 por gramo de MSd (p<0.05), pero no tuvo efecto estadístico sobre la concentración de AGV (p>0.05). Conclusiones. Bajo condiciones in vitro, con pH próximo a la neutralidad, la mayor edad del pasto reduce la DMS y aumenta la producción de CH4 por gramo de MSd, mientras que la reducción en la relación F/S aumenta la DMS y la producción de CH4 por gramo de MSd, último hallazgo que contrasta con los reportes in vivo.
Palabras clave: carbohidratos, digestibilidad in vitro, estado de madurez, producción de metano (Fuentes: CAB, USDA).
INTRODUCTION
Methane (CH4) is one of the main products of ruminal fermentation. It is considered a greenhouse gas (GHG) and represents between 2 and 15% of the energy consumed by ruminants (1). CH4 is produced in the rumen by a group of microorganisms of the Archaea domain, which use hydrogen (H2) to reduce carbon dioxide (CO2) to CH4, maintaining a low H2 pressure that favors fermentation processes (2).
Most dairy farms in Colombia are based on Kikuyu grass (Pennisetum clandestinum) as the main grazing grass for the animals. Average levels of neutral detergent fiber (NDF), nonstructural carbohydrates (NSC) and crude protein (CP) in Kikuyu are 58.1, 13.4, and 20.5%, respectively (on a dry matter bases (DM)) and fluctuate depending on the agronomic management (3). Because of its composition, Kikuyu grass presents nutritional constraints affecting milk yield and quality. Thus, supplements are commonly offered to improve the availability of NSC and energy in the rumen.
Feeding concentrate supplements to grazing ruminants is associated with a reduction of CH4 emissions. A change in the relationship feed: concentrate (DM bases) from 47: 53 to 68: 32 increased CH4 emissions from 538 to 648 g/cow/day (4). Different carbohydrates change the volatile fatty acids (VFA) profile and the production of CH4. Cellulose and starch degradation leads to glucose formation, which can be fermented in different pathways resulting in varying amounts of H2. The production of CH4 per unit of food digested depends on the amount of H2 formed, according to the stoichiometric ratio 4H2+CO2→CH4+2H2O. The formation of acetate and butyrate results in 1 and 0.5 moles of CH4 per mole of fermented glucose, respectively. On the other hand, propionate formation requires a net input of reduced equivalents, decreasing CH4 production. Fiber fermentation results in the preferential production of acetate, while feeding NSC increases the proportion of propionate (2). The objective of this study was to determine the effect of Kikuyu grass (Pennisetum clandestinum) harvested at two different ages and three forage: concentrate supplement ratios (F/C) on methane (CH4) production, dry matter digestibility (DMD), and fermentation profile using the in vitro gas production technique.
MATERIALS AND METHODS
Location. The study was conducted at the Nutrition and Animal Feeding Laboratory facilities of Universidad de Antioquia, Medellin, Colombia.
Substrates and chemical analysis. The substrates tested were Kikuyu grass (Pennisetum clandestinum) and a commercial concentrate supplement formulated for dairy cows. The grass was harvested at Betania farm, owned by Solla SA Company, during vegetative stage by cutting it 10 cm above the ground at 30 and 60 days of age. This farm is located in Santa Rosa de Osos municipality (Ant., Colombia) at 2460 m.a.s.l., with 18°C average temperature (range: 4 to 27°C), 70% relative humidity and 2400 mm average annual precipitation, corresponding to a lower montane wet forest (LMWF). The pasture was managed under a rotational grazing system with chemical fertilization (190, 54, 16, and 9 kg N, P, K, and S/ha/year).
Grass samples were dried in forced air oven at 65°C for 48 hours. Grass and supplement samples were ground to 1 mm for chemical analysis including DM, CP, ether extract (EE), ash (5), NDF (6), acid detergent fiber (ADF), acid detergent lignin (ADL) (7) and gross energy (GE). The GE was assessed in an adiabatic bomb calorimeter (IKA C5000, Rhys International Ltd., United Kingdom). NSC values were obtained by the equation, NSC=100-(%CP+%EE+%Ash %NDF) (8). The chemical description of substrates is presented in table 1.
Experimental treatments. Six treatments, resulting from the combination of pasture age (30 to 60 days) and inclusion level of concentrate supplement (0, 25 and 50%) were evaluated in a 2x3 factorial design. The treatments were: K30-100/0, K30-75/25, K30-50/50, K60-100/0, K60-75/25, and K60-50/50. Descriptors K30 and K60 correspond to Kikuyu age, while 100/0, 75/25 and 50/50 to the F/C ratio (DM basis). Treatments were replicated three times, corresponding to three sources of ruminal inoculum, each obtained from a different animal.
Gas production technique. This technique was conducted following protocols by Posada et al (9). Grass and concentrate supplement were ground to 1 mm and added (0.5 g) to each flask (100 ml) according to the percentages set for each treatment.
The inocula were obtained from three Holstein Friesian adult cows from La Montaña farm, owned by Universidad de Antioquia (San Pedro de los Milagros, Ant., Colombia). The cows were grazing on 42 days old Kikuyu and were provided mineralized salt and water ad libitum. The inocula were collected in the morning (6:00 AM) and transported in thermos preheated with 40°C water to the laboratory, where they were filtered through a cotton mesh, gassed with CO2 and kept in a warm water bath during inoculation.
Preparation of the buffer solution followed recommendations by McDougall (10), using a 9/1 (45/5 ml/ml) buffer/inoculum ratio and (1M) citric acid was added to the mixture to obtain pH 6.5 (11). The flasks were sealed with rubber stoppers and stored in a forced-air oven at 39 °C throughout the fermentation process.
Total gas and CH4. Total gas and CH4 production was determined at 6, 12, 24 and 48 h of incubation. Gas pressure generated in the incubation flasks was measured with a digital transducer (Ashcroft 2089QG - Precision Digital Test Gauges, Stratford, CT, USA) and the PSI readings were converted to volume (ml) using the equation
Y = -0.1833 + 0.0598 X2 (9).
After reading the pressure, the generated gas was removed from the flasks using a plastic syringe and then stored in plastic bags for later CH4 analysis. For this purpose, gas samples (100 µl) were taken with a gas-tight syringe and manually injected into a gas chromatograph (Thermo TRACE GC UltraTM, Thermo Scientific, USA) equipped with a flame ionization detector (FID). The chromatographic conditions corresponded to a 30 m nonpolar column, 0.32 mm and 0.25 µm, 200°C injection port temperature, 50: 1 split injection mode, 250°C detector temperature, 30°C (5 min) up to 200°C (30°C/min) oven temperature, and helium as a carrier gas (1.0 ml/min). CH4 data were processed according to Lopez and Newbold (12). CH4 production (ml) was calculated from total gas volume (ml) and CH4 concentration. Gas and CH4 production (ml) was expressed per gram of incubated dry matter (iDM) and per gram of digestible dry matter (dDM). CH4 production was also expressed as a percentage of the incubated GE (iGE) and incubated digestible energy (iDE), giving CH4 a caloric value of 9.45 kcal/L (13).
Dry matter digestibility. DMD was determined gravimetrically at the times of gas pressure readings by filtering the incubation flasks contents using glass crucibles (porosity 1, 100-160 µm) and a vacuum pump. The residues were dried in the crucibles at 60°C for 48 hours in a forced-air oven and weighed.
Fermentation profile and pH. The pH and concentration of acetic, propionic and butyric acids were determined in the liquid after filtration. This liquid was preserved by adding sulfuric acid (98% v/v) until pH 2 was reached. The pH was determined with digital pH meter (Handylab pH 1, Schott® Instruments, SI Analytics GmbH, Germany) before acidification.
To determine VFA, liquid samples (1 µl) were injected into a gas chromatograph (Thermo TRACE GC UltraTM, Thermo Scientific, USA) equipped with a 30 m, 0.32 mm and 0.25 microns column. Temperatures were 300°C (FID), 210°C (inlet), and 68°C (1 min), ramp 10°C/min until reaching 140°C (1 min), ramp 40°C/min until reaching 200°C (1 min) (oven). The mode of injection was split and helium was used as a carrier gas (2.5 ml/min).
Statistical analysis. A repeated measures analysis was performed using PROC MIXED of SAS (14). The fixed effects of the model were grass, F/C, and incubation time; the random effect was the ruminal inoculum. Treatment means were compared using PROC LSMEANS of SAS (14).
RESULTS
Table 2 shows the chemical composition of the treatments. The 30 days-old grass had higher CP and NSC content, but lower NDF, ADF and ADL compared to 60 days. The NSC/NDF ratio increased as concentrate increased in the mix.
Tables 3 and 4 show that grass age significantly (p<0.05) affected DMD, cumulative production of gas and CH4 per gram of dDM, and the percentage of iDE transformed into CH4. Following 48 hours of incubation, the 30 days old grass showed greater DMD (68.97 vs. 65.30%), decreased production of gas (463.56 vs. 499.27 ml) and CH4 (101.37 ml vs. 109.97) per gram of dDM, and lower production of CH4 as a percentage of iDE (22.83 vs. 24.70%) compared with the 60 days grass.
The F/C ratio significantly affected all variables (p<0.05). The F/C presented a growing trend as grass was reduced and concentrate increased between 0 and 48 hours of incubation. The average CH4 production in this time frame, regardless of grass age, was 57.40, 73.70, and 81.85 ml (per gram of iDM) and 96.15, 107.15 and 113.70 ml (per gram of dDM) for 100/0, 75/25, and 50/50 F/C ratios, respectively. For the same ratios, the average CH4 production corresponded to 12.50, 16.50 and 18.80% of the iGE, and 21.30, 23.80 and 26.20% of the iDE, respectively. After incubation, DMD was 60.55, 68.85 and 72.00% for 100/0, 75/25 and 50/50 F/C ratios, respectively. Results behaved in a similar way when the means of different F/C rates were compared inside each grass age.
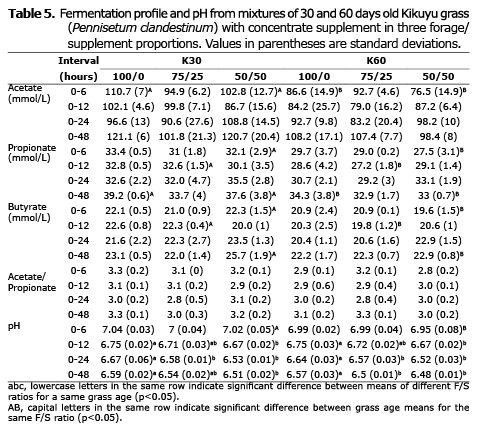
Table 5 shows that grass age significantly affected (p<0.05) acetic, propionic and butyric acid concentration, with the highest values for the young (30 days) compared with the 60 days old grass (117.87, 36.83 and 23.60 vs. 104.67, 33.40 and 22.47 mmol/L, respectively). The F/C ratio had no statistical effect on VFA concentration or the acetate/propionate ratio (A/P) (p>0.05), but it significantly affected pH (p<0.05). Treatments including more concentrate had lower pH values (6.50 and 6.52 for 50/50 and 75/25 ratios, respectively, compared with 6.58 for the 100/0 ratio). The grass age*F/C interaction was not significant (p>0.05) for the variables presented in tables 3, 4 and 5.
DISCUSSION
Effect of grass age on production of gas, CH4, and DMD. Caro and Correa (15) reported a 7.8% increase in NDF and 2.7% reduction in NSC for Kikuyu grass harvested at 32 vs. 58 days of regrowth. In the present study, NDF increase and NSC reduction exceeded the figures reported by those researchers (Table 1). It is expected that forage quality decrease with maturity, which was verified by Caro and Correa (15), who found that changes in the chemical composition affect in situ digestibility at 24h incubation. They reported a 6.7% increase in grass digestibility after 32 days of regrowth compared to 58 days. This is in agreement with the present study, where DMD at 24 hours of incubation was higher for the younger grass (Table 3). Within species, increased harvest age is associated with higher NDF, ADF and ADL levels, and lower CP content (Table 1) as well as DMD reduction.
Chaves et al (16) concluded that CH4 produced by grazing heifers was significantly affected by the type of grass, reflecting differences in maturity and chemical composition and confirming that CH4 production is primarily a function of the amount of cell wall digested in the rumen. In the present study, pasture age significantly affected gas and CH4 production (per gram of dDM) and CH4 (as percent of iDE) (Tables 3 and 4). According to Sun et al (17), CH4 production should be expressed in relation to dDM, instead of iDM. They reported that Cichorium intybus and Lolium perenne fodder had similar CH4 production on iDM basis despite their differences in NDF (28.1 and 49.9%, respectively) and the higher digestibility of Cichorium intybus. In the present study, treatment K30-100/0 resulted in lower NDF, ADF and ADL (Table 2), higher accumulated digestibility at 48 hours (p<0.05), and similar gas and CH4 production (per gram of iDM) compared to treatment K60-100/0. However, when gas production and CH4 were expressed per gram of dDM (Tables 3 and 4) the values were statistically higher for the older grass (60 days) (p<0.05). Accordingly, highly digestible forage can produce the same gas and CH4 per gram of iDM; which switches when production of both is expressed as per gram of dDM. The higher digestibility of the substrate mathematically reduces gas and CH4 production and biologically increases YATP. Van Soest et al (18) defined microbial efficiency as the proportion of the substrate energy fixed by the cells and, thus, it is inversely related to VFA and CH4 production.
Effect of F/C ratio on DMD, CH4, and gas production. The increase in gas production and CH4 per gram of iDM observed with decreasing F/C ratios (Tables 3 and 4) can be attributed to higher NSC vs. NDF in the incubated substrate (Table 2). The high NSC proportion in the concentrate supplement (Table 1) explains its upward trend as F/C decreased, resulting in a higher NSC/NDF ratio (Table 2). Pellikaan et al (19) also found increased in vitro CH4 and gas production from substrates with low NDF and high starch content. They obtained 399.9 and 66.8 ml for gas and CH4, respectively, from potato starch (per gram of incubated organic matter); while values were 273.3 and 47.6 ml, respectively, from grass silage. Navarro-Villa et al (11) also reported increased production of gas and CH4 for incubated barley grain compared with barley straw and grass silage. In the present study, the increased gas and CH4 production from substrates with concentrate supplement can be explained by their higher DMD (Table 3). However, it is important to note that this increased digestibility was not sufficient to generate a downward trend in gas and CH4 output (at 48 hours of incubation) as F/C decreased when they were expressed as per gram of dDM. The only exception to this was gas production per gram of dDM from grass harvested at 60 days (Tables 3 and 4). Lower levels of incubated protein as F/C decreased -especially for 30 days grass- may be associated with reductions in CH4. According Inthapanya and Preston (20), less protein degradation in the incubated substrate decreases CH4 production. However, this effect was smaller in the present study compared to that generated by a higher NSC/NDF ratio, as previously stated.
Increased in vitro CH4 production per gram of iDM and dDM in treatments with low F/C (Table 4) is not in agreement with in vivo results (21), where it is accepted that rapidly fermenting diets decrease CH4 production. The effect of these diets on in vivo CH4 production is explained by a decrease in ruminal pH, affecting the growth of methanogens, protozoa (22) and cellulolytic bacteria (23), and increasing passage rate, which reduces the number of protozoa and thus interspecies transfer of H2 and methanogenesis (24). The in vitro gas technique does not allow replacing the liquid and/or solid fractions, and a buffer solution is added to guarantee pH stability between 6.5 to 7.0 (Table 5). Both things may explain the divergence between in vitro and in vivo results regarding CH4 for increasing NSC levels. This was reported by Navarro-Villa et al (11), who measured 70% higher CH4 for grains compared with forages incubated in vitro, while McGeough et al (25) reported lower CH4 production from ruminants fed cereal based diets.
The CH4 increase, as a percentage of the iGE and iDE, is consistent with CH4 emissions per gram of iDM and dDM (Table 4). Values ranged from 8.8 (K60-100/0) and 15.1% (K60-50/50) of iGE after 24 hours of incubation, surpassing the results by Bhatta et al (26) who evaluated mixed diets also at 24 hours of incubation and obtained between 4.4 and 7.8% of GE.
Effect of grass age on fermentation profile. Glucose is a common intermediary among carbohydrates degraded in the rumen and their final products (VFA, CO2 and CH4). The mechanism that controls glucose partition between different VFA depends on food composition (2). Tropical grasses usually grow and mature faster, rapidly turning into fibrous and less digestible. The lower NDF content (Table 2) and the larger DMD (Table 3) of 30-day grass treatments is consistent with the higher VFA concentration, as reported in Table 5, explaining the significant effect of age on acetate, propionate and butyrate production (p<0.05).
Effect of F/C ratio on fermentation profile. When fiber-rich diets change to diets based on concentrates, a decrease can be expected in ruminal pH (27), number of fibrolytic bacteria (23) and the A/P ratio (28). Although in the present study F/C significantly affected the pH, it tended to remain high and stable due to the HCO3-/CO2 balance of the buffer solution, which could explain the lack of significant effect of the F/C ratio on the A/P ratio (Table 5). Citric acid has been suggested to overcome the buffer effect and perform in vitro evaluations at pH lower than 6 (11). However, that strategy did not work in the present study; pH readings at 6 hours of incubation (Table 5) were higher than those recorded at the beginning (pH 6.5). This could be explained by citric acid being rapidly fermented by rumen microorganisms to CO2 and acetic acid (29).
The in vitro gas technique does not remove VFA via absorption, so its concentration depends on the fermentation rate. Since DMD increases with incubation time, a higher VFA concentration is expected as fermentation continues. However, this was not observed in this study (Table 5). It can be assumed that microorganisms degraded VFA to obtain energy, as observed by Wang et al (30), which would explain the stability of its concentration over the incubation time.
The results of this study confirm that grass age affects DMD, CH4, gas production, and fermentation profile due to a higher proportion of cell wall. A reduction of F/C ratio simultaneous with a high NSC/NDF ratio improves substrate digestibility and increases CH4 and gas production, contradicting the in vivo reports. The pH, which is controlled by the buffer solution, hinders the study of the effect of F/C ratio on methanogenesis in in vitro systems. Therefore, for future studies we suggest to change the formulation of the buffer solution to enable the assessments of mixed diets at a pH close to in vivo.
REFERENCES
1. Kumar S, Puniya AK, Puniya M, Dagar SS, Sirohi SK, Singh K et al. Factors affecting rumen methanogens and methane mitigation strategies. World J Microbiol Biotechnol 2009;25(9):1557-1566. [ Links ]
2. Janssen PH. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim Feed Sci Tech 2010; 160(1-2):1-22. [ Links ]
3. Correa HJ, Pabón ML, Carulla JE. Valor nutricional del pasto kikuyo (Pennisetum clandestinum Hoechst Ex Chiov.) para la producción de leche en Colombia (Una revisión): I-Composición química y digestibilidad ruminal y posruminal. Livest Res Rural Dev 2008; 20(4) [en línea] [fecha de acceso 18 de julio de 2014]. URL disponible en: http://www.lrrd.org/lrrd20/4/corra20059.htm. [ Links ]
4. Aguerre MJ, Wattiaux MA, Powell JM, Broderick GA, Arndt C. Effect of forage-to-concentrate ratio in dairy cow diets on emission of methane, carbon dioxide, and ammonia, lactation performance, and manure excretion. J Dairy Sci 2011;94(6):3081-3093. [ Links ]
5. Association of Official Analytical Chemist - AOAC. Official Methods of Analysis. 18 ed. Gaithersburg, MD: AOAC Int, 2011. [ Links ]
6. Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 1991; 74(10):3583-3597. [ Links ]
7. Raffrenato E, Van Amburgh ME. Technical note: Improved methodology for analyses of acid detergent fiber and acid detergent lignin. J Dairy Sci 2011; 94(7):3613-3617. [ Links ]
8. Detmann E, Valadares Filho SC. On the estimation of non-fibrous carbohydrates in feeds and diets. Arq Bras Med Vet 2010; 62(4):980-984. [ Links ]
9. Posada SL, Noguera R, Bolívar D. Relación entre presión y volumen para la implementación de la técnica in vitro de producción de gases. Rev Colomb Cienc Pecu 2006; 19(4):407-414. [ Links ]
10. McDougall EI. Studies on ruminant saliva. 1. The composition and output of sheep's saliva. Biochem J 1948; 43(1):99-109. [ Links ]
11. Navarro-Villa A, O'Brien M, López S, Boland TM, O'Kiely P. Modifications of a gas production technique for assessing in vitro rumen methane production from feedstuffs. Anim Feed Sci Tech 2011; 166-167:163-174. [ Links ]
12. López S, Newbold CJ. Measuring methane production from ruminants. The Netherlands: Springer; 2007.
13. Nkrumah JD, Okine EK, Mathison GW, Schmid K, Li C, Basarab JA et al. Relationships of feedlot feed efficiency, performance, and feeding behavior with metabolic rate, methane production, and energy partitioning in beef cattle. J Anim Sci 2006; 84(1):145-153. [ Links ]
14. SAS/STAT [programa de ordenador]. Versión 9.1.3. Cary (NC): SAS Institute Incorporation; 2003. [ Links ]
15. Caro F, Correa HJ. Digestibilidad posruminal aparente de la materia seca, la proteína cruda y cuatro macrominerales en el pasto kikuyo (Pennisetum clandestinum) cosechado a dos edades de rebrote. Livest Res Rural Dev 2006; 18(10) [en línea] [fecha de acceso 22 de agosto de 2014].URL disponible en: http://www.lrrd.org/lrrd18/10/caro18143.htm. [ Links ]
16. Chaves AV, Thompson LC, Iwaasa AD, Scott SL, Olson ME, Benchaar C et al. Effect of pasture type (alfalfa vs. grass) on methane and carbon dioxide production by yearling beef heifers. Can J Anim Sci 2006; 86(3):409-418. [ Links ]
17. Sun XZ, Hoskin SO, Muetzel S, Molano G, Clark H. Effects of forage chicory (Cichorium intybus) and perennial ryegrass (Lolium perenne) on methane emissions in vitro and from sheep. Anim Feed Sci Tech 2011; 166-167:391-397. [ Links ]
18. Van Soest PJ, France J, Siddons RC. On the steady-state turnover of compartments in the ruminant gastrointestinal tract. J Theor Biol 1992; 159(2):135-145. [ Links ]