Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268
Rev.MVZ Cordoba vol.20 supl.1 Córdoba Dec. 2015
ORIGINAL
Clinical safety of dichlorvos (45%), cypermethrin (5%) and piperonyl butoxide (25%) administered by spray on the skin of cattle
Seguridad clínica del diclorvos (45%), cipermetrina (5%) y butóxido de piperonilo (25%) administrado por aspersión sobre la piel de bovinos
Alessandra C Moraes,1 M.Sc, Ed JR Prado,2 M.Sc, Vanessa P Faria,1 MV, Thais MS Gírio,3 Ph.D, Wilson G Manrique,2 Ph.D, Marco AA Belo,1,4* Ph.D.
1 São Paulo State University - UNESP, Department of Preventive Veterinary Medicine, Via de acesso Prof. Paulo Donato Castellane s/n CEP 14884-900, Jaboticabal/SP - Brazil. São Paulo State University - UNESP, Brasil.
2 Department of Veterinary Pathology, Via de acesso Prof. Paulo Donato Castellane s/n CEP 14884-900, Jaboticabal/SP - Brazil.
3 Uzinas Chimicas Brasileiras S.A. Deparment of Research and Technical Development, Praça Dr. Joaquim Batista, 150, Centro 14870-090, Jaboticabal/SP - Brazil.
4 Camilo Castelo Branco University, Laboratory of Animal Pharmacology and Toxicology. Av. Hilário da Silva Passos, 950. Parque Universitário CEP. 13690-000, Descalvado/SP - Brazil.
*Correspondencia: maabelo@hotmail.com
Received: October 2014; Acepted: March 2015.
ABSTRACT
Objective. Due to the importance of controlling ectoparasites, associated with the necessity of technical knowledge on the safety of topical treatment with organophosphates, pyrethroids and piperonyl butoxide to the animal organism, this bioassay was carried out to evaluate the clinical safety of the association of dichlorvos (45%) + cypermethrin (5%) + piperonyl butoxide (25%) administered by spray on the skin of cattle, through the study of clinical parameters, biochemical, haematological and behavioral changes. Materials and methods. Sixteen crossbred animals with a mean age of 18 months, males and females grouped into two treatments with eight animals each: T1 (1:800 v/v) and T2 (1:200 v/v). Were collected blood samples at six different times: before treatment (BT), 24, 48, 72, 96 and 192 hours post treatment (HPT). Results. The antiparasitic association administered by spray on the skin did not result in changes in the enzymatic activity of ALT, AST, GGT and ALP, as well as in serum albumin, triglycerides, cholesterol, urea and creatinine, demonstrating the safety of this antiparasitic compound for maintaining hepatic and renal functionality. The erythrocyte, leukocyte and platelet studies showed no changes caused by treatments, and no clinical signs and behavioral changes were observed after treatment. Conclusions. These findings demonstrated good safety margin for spray treatment on the skin with this antiparasitic compound, even when administered at a dilution of 1:200 v/v, which is four times the dose recommended for ectoparasite control.
Key words: Clinical safety, bovine, organophosphates, piperonyl butoxide, pyrethroids, spray (Source: DeCS, USDA).
RESUMEN
Objetivo. Debido a la importancia del control de ectoparásitos en bovinos, asociado a la necesidad de conocimientos técnicos sobre la seguridad del tratamiento tópico con organofosforados, piretroides y butóxido de piperonilo, se realizó este bioensayo para la evaluación de la seguridad clínica de la asociación de diclorvos (45%) + cipermetrina (5%) + butóxido de piperonilo (25%), administrado por aspersión en la piel del ganado bovino, a través del estudio de los parámetros clínicos, bioquímicos, hematológicos y comportamentales. Materiales y métodos. Dieciséis animales entre machos y hembras cruzados con edad media de 18 meses, agrupados en dos tratamientos de ocho animales cada uno: T1 (1:800 v/v) y T2 (1:200 v/v). Fueron colectadas muestras de sangre en seis momentos diferentes: antes del tratamiento (BT), 24, 48, 72, 96 y 192 horas post tratamiento (HPT). Resultados. La asociación antiparasitaria administrada por aspersión en la piel no alteró la actividad enzimática de ALT, AST, GGT y FA, así como la albúmina, triglicéridos, colesterol, urea y creatinina, que demuestra la seguridad de este compuesto antiparasitario en la función renal y hepática. El análisis de eritrocitos, leucocitos y plaquetas no mostraron cambios en los tratamientos, tampoco fueron observados signos clínicos y de comportamiento post tratamiento. Conclusiones. Estos resultados demostraron buen margen de seguridad en el tratamiento por aspersión en la piel con este compuesto antiparasitario, incluso cuando se administra en una dilución de 1:200 v/v, que es cuatro veces la dosis recomendada para el control de ectoparásitos.
Palabras clave: Aspersión, bovino, butóxido de piperonilo, organofosfatos, piretroides, seguridad clínica (Fuente: DeCS, USDA).
INTRODUCTION
The South America assumes an important position as a world producer of beef, contributing to economic and social development, responsible for thousands of direct and indirect jobs (1). According to Soares et al (2), the development of the cattle industry depends on the health herd management, and the control of parasitic diseases is revealed as an essential factor to make this activity more effective and competitive.
The ectoparsitoses are responsible for high economic losses to the cattle industry in regions with tropical and subtropical climates (3). However, there are a number of active principles that participate in chemotherapy formulations used for parasite control, and the administration of them without epidemiological criteria and incorrect doses, may compromise the therapeutic efficacy and the health of cattle (4).
The animals are constantly exposed to many potentially toxic substances, such as pesticides, spoiled food, industrial waste, toxic plants, among others (5). Most of these chemical compounds induce lipid peroxidative processes mediated by the action of free radicals, resulting in damage to biomembranes, consequently promoting cellular and tissue dysfunction (6).
In cases of poisoning is high the occurrence of hepato-renal syndromes. According to Varga and Puschner (7) and Belo et al (8), severe damage to liver by different etiologies can cause changes in renal function, aggravating the clinical condition of the animal. In this context, haematological and serum biochemical analysis provide important information in the diagnosis and prognosis of morbid conditions in animal populations (9).
Another extremely important aspect is related to the safety of exogenous administration of pharmacological compounds. In addition to their therapeutic activity, these formulations should provide clinical safety to animals, considering that every substance is potentially harmful to the organism, the right dose differentiates the medicine from the toxicant (10).
Considering the great importance of controlling ectoparasites in cattle, associated with lack of technical information on the safety of topical treatments with organophosphates, pyrethroids and piperonyl butoxide to the animal organism, this study aimed to evaluate the clinical safety of dichlorvos (45%)+ cypermethrin (5%) + piperonyl butoxide (25%) after topical administration by spray, through the study of evolution of the clinical and toxicological changes.
MATERIALS AND METHODS
Animals. Sixteen crossbred animals with a mean age of 18 months, males and females, were divided into two treatments with eight animals each: T1 (treated with the dose recommended by parasite control at a dilution of 1:800) and T2 (treated with four times the recommended dose, dilution of 1:200). After treatment, both groups were kept in different pastures in Estância Modelo Farm, City of Descalvado, São Paulo State, Brazil, during the period of analysis. The experimental design is in accordance with the Guideline for Good Clinical Practice described by McGroth et al (11), and was approved by the UNICASTELO Institutional Ethics Committee with the process number 2517-2737/09.
Experimental Design. The 16 bovines were sampled at six different times: before treatment (BT) to obtain the basal values (standard physiology), 24, 48, 72, 96 and 192 hours post treatment (HPT). This experimental model allows the evaluation of the toxicological and clinical evolution of animals after treatment.
Antiparasitic treatment. The treatments were performed in covered paddock, being sprayed five liters of antiparasitic (Colpo 75®, Cypermethrin 5% + 45% Dichlorvos + 25% butoxide Piperolina - Uzinas Chimicas Brasileiras Company) per animal, in two treatments with eight animals each: T1 (dilution of 1:800) and T2 (dilution of 1: 200).
Haematological study. Sampling of blood for clinical and haematological evaluation were performed by jugular vein puncture, and aliquoted two sets of syringes of 5 mL (40x16 needles), and the first “set” was used 50 µL of EDTA anticoagulant for collection of the plasma and the second without anticoagulant to obtain serum. For determination of erythrocyte was used automatic blood cell counter (Model CC510, Celm). The counting of leukocytes and platelets was performed manually in a Neubauer chamber. The percentage of packed cell volume was determined by micro-capillary hematocrit tubes, centrifuged 5 minutes 600 x g. At the time of blood collection, small samples were aimed at making blood extensions for differential leukocyte counts. After staining with May-Grünwald-Giemsa-Wright, was performed by counting 200 cells, establishing the percentage of each cell type (12).
Serum biochemical. Blood samples without anticoagulant were centrifuged at 500 x g/10 minutes, the serum was separated for determining levels of aspartate aminotrasnferase (AST), alanine aminotransferase (ALT), gamma glutamyl transferase (GGT), alkaline phosphatase (ALP), albumin , urea, creatinine, total and direct bilirubin, triglycerides and cholesterol, performed enzymatic and colorimetrically in a semi-automatic biochemical analyzer, model LabQuest.
Clinical and behavioral evaluation. During the experimental period, the possible occurrences of clinical and behavioral changes in animals subjected to treatment with the antiparasitic compound were observed. Physiological parameters such as heart frequency, respiratory frequency, body temperature and capillary perfusion time were measured at the time of blood collection.
Statistical analysis. The data was subjected to analysis of variance using completely randomized split plot design, the main treatments of the experimental groups (two treatments) and the subplot experimental periods (six periods), using the PROC MIXED procedure in the SAS program, version 8.2 (13). Multiple comparisons were assessed by the Tukey test at 95% confidence level according to Snedecor and Cochran (14).
RESULTS
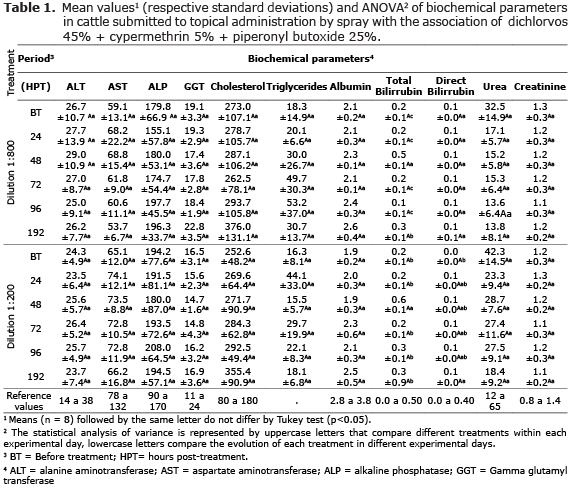
In serum-biochemical study of the cattle treated with antiparasitic association, no significant changes (p>0.05) in serum enzyme activity of ALT, AST, GGT and ALP were observed in bovines treated with a dilution of 1:800 (T1) when compared to animals subjected to a dilution of 1:200 (T2), as well as the comparison of clinical outcome of animals in each treatment (Table 1).
In the study of liver function, bovines treated with both doses presented no significant changes (p<0.05) in serum determination of triglycerides, cholesterol, albumin, total and direct bilirubin (Table 1). Likewise, there were no significant changes in serum levels of these biochemical parameters when comparing the controls values measured before treatment related to different times after treatment. Only total and direct bilirubin were significantly increased (p<0.05) 48 h after treatment in both concentrations (Table 1). Other relevant data shown in table 1 refers to normal values in blood urea and creatinine, in all analyzed periods.
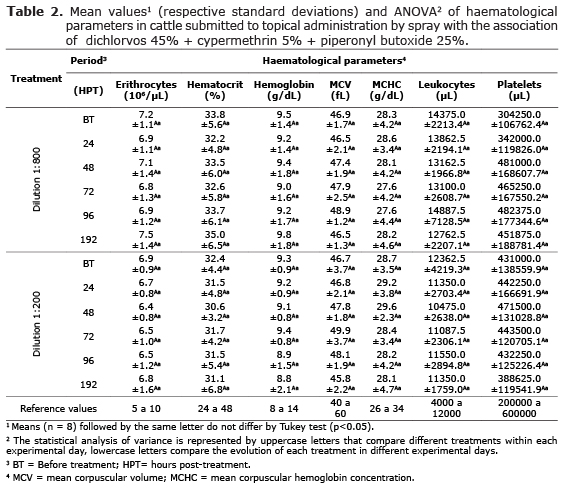
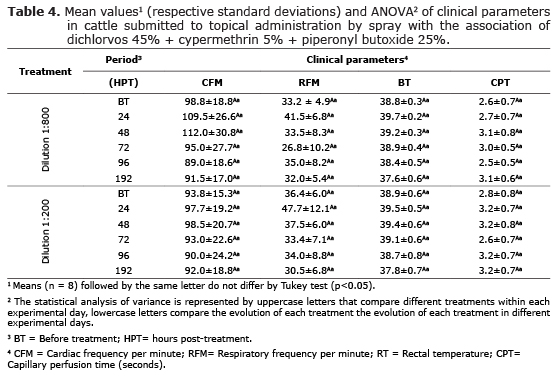
In erythrocyte study (Table 2), bovines treated with both dilutions showed no significant changes (p>0.05) in the number of erythrocytes, percentage of hematocrit, mean corpuscular volume (MCV), hemoglobin levels and mean corpuscular hemoglobin concentration (MCHC), as well as the comparison of the clinical evolution of animals in each treatment. Similarly, platelet and leukocyte study (Table 2) presented no significant changes (p> 0.05) between the animals throughout the experimental period, including differential leukocyte count (Table 3) that showed no significant changes (p>0.05) in the absolute and relative number of circulating neutrophils, lymphocytes, monocytes, eosinophils and basophils in cattle treated with both concentrations of 1:800 v/v and 1:200 v/v.
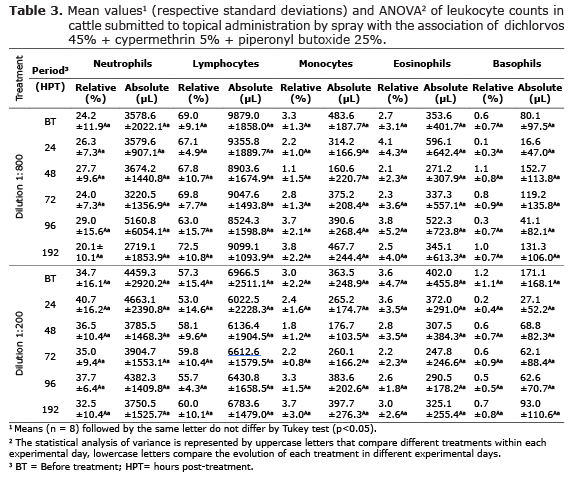
The behavioral evaluation and clinical signs of the cattle treated (Table 4) showed no changes that could suggest the occurrence of toxic effects resulting from the use of this antiparasitic compound when sprayed at concentrations of 1:800 v/v and 1:200 v/v. However, it was found transitory and no significant increase (p>0.05) in body temperature, cardiac frequency and respiratory frequency 24 hours after treatment.
DISCUSSION
The results of serum-biochemical study demonstrated the clinical safety of this compound which did not cause significant changes in permeability of the cell membrane of hepatocytes, or even lysis these cells. Tests for serum enzyme activity of ALT, AST, LDH, ALP and GGT are indicative of hepatocellular injury and cholestatic changes, while the functional tests determine the concentration of substances synthesized in the liver or belonging to metabolic processes, such as albumin, urea, glucose, cholesterol, triglycerides, among other (15, 16).
Bovines treated with both doses presented no significant changes in serum determination of triglycerides, cholesterol and albumin. These results suggest the maintenance of liver functions. Changes in energy metabolism are generally present in acute liver failure (17). Moreover, the high concentration of glucocorticoids due to handling stress and treatment could promote energy metabolism by stimulating gluconeogenesis, increasing the bioavailability of free fatty acids resulting from the breakdown of triglycerides (15). Only total and direct bilirubin increased 48h after treatment in both concentrations. However, the current mean values of 0.57 and 0.62 mg/dL observed in animals treated with dilutions of 1: 800 and 1: 200, respectively, are close to the reference values for the species. According to Ohgi et al (18), serum total bilirubin up to 50 mg/dL is considered normal for cattle. Bilirubin is a byproduct of the breakdown of heme molecule present in red blood cells, and hyperbilirubinemia can be primarily resulting from the destruction of red blood cells or liver dysfunctions.
The transitory increase in serum bilirubin levels observed 48 hours after treatment showed no positive correlation with haematological parameters that showed complete normal in relation to the number of circulating erythrocytes. Likewise, hyperbilirubinemia induces jaundice, and this clinical sign was not observed in the examination of the animals. On the other hand, normal values in blood urea and creatinine was observed in all analyzed periods, indirectly demonstrating the clinical safety of the treatments on the process of excretion of these substances by the kidneys, as well as in protein catabolism in hepatic tissues, responsible for producing large part of the circulating urea (7).
In cattle treated with both concentrations of 1:800 v/v and 1:200 v/v no significant changes were observed in erythrocyte, platelet and leukocyte studies, at the same time, the mean values observed were within of the normal range described for bovines according to Grunwaldt et al (19). Therefore, these results demonstrated the clinical safety of this antiparasitic formulation, even when applied at a concentration four times greater the dose recommended for parasite control. Other studies demonstrated the clinical safety and efficacy of antiparasitic treatment administered through skin route, White et al (20) demonstrated the persistent efficacy of spinosad administered by spray on the skin in natural infestations of chewing and sucking lice on cattle and Davey et al (21) observed the efficacy of a single wholy-body treatment of spinosad against ticks (Rhipicephalus Boophilus microplus) on cattle, in both studies were not reported the occurrence of side effects of drug therapy.
Despite the lipophilic characteristic of many active principles, little is known about the absorption, systemic toxicity and side effects of drugs administered by spray on the skin to control ectoparasites (22). The treatment with this antiparasitic compound when sprayed on the skin at concentrations of 1:800 v/v and 1:200 v/v do not result in behavioral and clinical changes. However, it was found transitory increase in body temperature, cardiac frequency and respiratory frequency 24 hours after treatment. A hypothesis which explains these findings relates to the occurrence of stress, resulting from the containment and treatment. The organic response to stress conditions is complex, involving neuro-endocrine mechanisms with the release of cortisol and catecholamines (23) that act by increasing the metabolic activity and therefore heat production, as well as cardiac and respiratory function (24).
Bovines treated with both dilutions did not present ataxia, salivation, jaundice, inappetence, among others, which could characterize intoxication throughout the experimental period, demonstrating the safety of the treatments, even when administered at a dilution of 1:200 v/v. Therefore, the results in the studies of clinical parameters, biochemical, haematological and behavioral evaluation demonstrated good safety margin for spray treatment on the skin with this antiparasitic compound (dichlorvos 45%+cypermethrin 5% + piperonyl butoxide 25%) in cattle.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this article.
REFERENCES
1. Macedo MCM. Integração lavoura e pecuária: o estado da arte e inovações tecnológicas. Rev Bras Zoot 2009; 38 (Supl. ):133-146. [ Links ]
2. Soares VE, Belo MAA, Rezende PB, Soccol VT, Fukuda RT, Oliveira GP, Costa AJ. Distribuition of Taenia saginata metacestodes: comparison of routine meat inspection and carcase dissection results in experimentally infected calves. Annals Trop Med Parasitol 2011; 105(5):393-401. [ Links ]
3. Soares VE, Belo MAA, Souza LM, Guiaro CR, Bortoletto-Neto O, Girio TMS. Associação de cipermetrina, diclorvos e butóxido de piperolina contra Rhipicephalus (Boophilus) microplus em bovinos naturalmente infestados. Arch Vet Sci 2009; 14(1):1-8. [ Links ]
4. Belo MAA, Prado EJR, Soares VE, Souza LM, Mota FCC, Giamlorenço TF, Gírio TMS. Eficácia de diferentes formulações no controle da mosca Haematobia irritans em bovinos naturalmente infestados. Biosci J 2012; 28(2):245-250. [ Links ]
5. Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: A review. Liver Transplant 2005; 11:1031-1047. [ Links ]
6. Mudron P, Rehage J, Qualmann K, Sallmann HP, Scholz H. A study of lipid peroxidation and vitamin E in dairy cows with hepatic insufficiency. J Vet Med A 1999; 46:219-224. [ Links ]
7. Varga A, Puschner B. Retrospective study of cattle poisonings in California: recognition, diagnosis, and treatment. Vet Med: Res Reports 2012; 3:111-127. [ Links ]
8. Belo MAA, Souza LM, Soares VE, Sobreira MFR, Cassol DMS, Toma SB. Tratamento hepatoprotetor favorece a resposta leucocitária de ratos Wistar intoxicados por CCL4. Arch Vet Sci 2009; 14(2):74-82. [ Links ]
9. Belo MAA, Souza LM, Soares VE, Sobreira MFR, Cassol DMS, Toma SB. Hepatoprotective treatment attenuates oxidative damages induced by carbon tetrachloride in rats. Exp Toxicol Pathol 2012; 64(3):155-165. [ Links ]
10. Melo DO, Ribeiro E, Storpirtis S. A importância e a história dos estudos de utilização de medicamentos. Rev Bras Cienc Farm 2006; 42(4):475-485. [ Links ]
11. McGroth JC, Drummond GB, McLachlon EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the arrive guidelines. Brit J Pharmacol 2010; 160(7):1573-1576. [ Links ]
12. Belo MAA, Souza DGF, Faria VP, Prado EJR, Moraes FR, Onaka EM. Haematological response of curimbas Prochilodus lineatus, naturally infected with Neoechinorhynchus curemai. J Fish Biol 2013; 82(4):1403-1410. [ Links ]
13. SAS Institute Inc. SAS/STAT software changes and enhancements though [computer program]. Release 8.2. Cary: SAS Institute; 2001. [ Links ]
14. Snedecor GW, Cochran WG. Statistical Methods. Iowa State University Press, Iwoa, USA, 1980. [ Links ]
15. Scarpelli LC, Belo MAA, Souza LM, Sabatini GA, Costa AJ, Moraes FR. Parâmetros hematológicos e bioquímicos de bovinos submetidos a diferentes doses de sulfóxido de albendazole, ivermectin e abamectin. ARS Veterinária 1999; 15:57-62. [ Links ]
16. Bode G, Young JW, Beitz DC. Invited review: Pathology, etiology, prevention and treatment of fatty liver in dairy cows. J Dairy Sci 2004; 87(10):3015-3124. [ Links ]
17. Mathur S, Constable PD, Eppley RM, Waggoner AL, Tumbleson ME, Haschek WM. Fumonisin B1 is hepatotoxic and nephrotoxic in milk-fed calves. Toxic Sci 2001; 60:385-396. [ Links ]
18. Ohgi T, Kamimura S, Minezaki Y, Takahashi M. Relationship between fat accumulation in the liver and energy intake, milk fat yield and blood metabolites in dairy cows. Anim Sci J 2005; 76(6):549-557. [ Links ]
19. Grunwaldt EG, Guevara JC, Estevez OR, Vicente A, Rouselle H, Alcuten N, Aguerregaray D, Stasi CR. Biochemical and haematological measurements in beef cattle in Mendonza plain Rangelands (Argentina). Trop Anim Health Prod 2005; 37(6):527-540. [ Links ]
20. White WH, Hutchens DE, Jones C, Firkins LD, Paul AJ, Smith LL, Snyder DE. Therapeutic and persistent efficacy of spinosad applied as a pour-on or a topical spray against natural infestations of chewing and sucking lice on cattle. Vet Parasitol 2007; 143(3-4):329-336. [ Links ]
21. Davey RB, George JE, Snyder DE. Efficacy of a single whole-body spray treatment of spinosad, against Boophilus microplus (Acari: Ixodidae) on cattle. Vet Parasitol 2001; 99(1): 41-52. [ Links ]
22. Beyene T, Tesega B. Rational veterinary drug use: Its significance in public health. J Vet Med Anim Health 2014; 6(12):302-308. [ Links ]
23. Belo MAA, Schalch SHC, Moraes FR, Soares VE, Otoboni AM, Moraes JRE. Effect of dietary supplementation with vitamin E and stoking density on macrophage recruitment and giant cell formation in the teleost fish, Piaractus mesopotamicus. J Comp Pathol 2005; 133(2-3):146-154. [ Links ]
24. Sordillo LM, Aitken SL. Impact of oxidative stress on the health and immune function of dairy cattle. Vet Immunol Immunopathol 2009; 128(1-3):104-109. [ Links ]