Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268
Rev.MVZ Cordoba vol.20 supl.1 Córdoba Dec. 2015
ORIGINAL
Effect of the diet traditional and non-traditional on the respiration and excretion in larvae of white shrimp Litopenaeus vannamei
Efecto de la dieta tradicional y no tradicional en la respiración y excreción de larvas de camarón blanco Litopenaeus vannamei
María Alejandra Medina-Jasso,1 M.Sc, Juan Francisco Arzola-González,2 Ph.D, Pablo Piña-Valdez,1,2* Ph.D, Mario Nieves-Soto,1,2 Ph.D.
1 Universidad Autónoma de Sinaloa, Programa Regional para el Doctorado en Biotecnología. Facultad de Ciencias Químico-Biológicas. Av. de Las Américas y Blvd. Universitarios s/n. Ap. Postal 1354. Culiacán, Sinaloa, México.
2 Universidad Autónoma de Sinaloa, Facultad de Ciencias del Mar. Av. Paseo Claussen s/n. Ap. Postal 610. Mazatlán, Sinaloa, México.
*Correspondence: marcos.meneses@anahuac.mxpablopina@live.com.mx
Received: August 2014; Acepted: February 2015.
ABSTRACT
Objective. It was studied the respiration and ammoniacal excretion of zoeas and mysis of Litopenaeus vannamei fed with the diet used traditionally (of microalgae and nauplios of Artemia) and another alternative (not traditional) of microalgae with rotifers. Materials and methods. After four hours the oxygen consumption and ammonia excretion in BOD bottles with 60 larvae (closed respirometers) was estimated. The concentrations of O2 and NH4+ were measured with an electrode polarográfico in the first case and with the indophenol blue technique for the second. Results. In zoea, oxygen consumption increased with development and showed statistical differences (p=0.023). In mysis, the oxygen consumption were significance in the traditional diet, whereas no differences were alternative (p=0.003). In both stages for the ammoniacal excretion increased development stage and there were detected statistical difference (p<0.001), although to the diets were not noticed significant differences. Conclusions. A higher energy absorption for zoea (I, II y III) what mysis (I, II y III) larvae was obtained, this is likely an interaction between rates of respiration and excretion caused by variations in the efficiency of absorption by the larvae. The weights obtained in both larvae were not supplied with differences between diets.
Key words: Ammonia, brachionus plicatilis, oxygen, white shrimp (Fuente: MeSH).
RESUMEN
Objetivo. Se analizó la respiración (O2) y excreción amoniacal (NH4+) en larvas zoea y mysis de camarón blanco Litopenaeus vannamei, alimentadas con las dietas tradicionales (microalgas y nauplios de Artemia) y no tradicionales (microalgas y rotíferos). Materiales y métodos. A las cuatro horas de experimentación se estimó el consumo de oxígeno y la excreción de amonio en botellas BOD con 60 larvas (respirómetros cerrados). La concentración de O2 se midió con un electrodo polarográfico y la NH4+ se determinó con la técnica de azul de Indofenol. Resultados. En zoea, el consumo de oxígeno incrementó con el desarrollo y se presentaron diferencias estadísticas (p=0.023). En mysis, los consumos de oxígeno presentaron una significancia entre la dieta tradicional, mientras en la alternativa no se obtuvieron diferencias (p=0.003). La excreción en ambos estadios larvales aumentó con la fase y se detectaron diferencias estadísticas (p<0.001), aunque no se registraron diferencias significativas en las larvas respecto a las dietas suministradas. Conclusiones. Se obtuvo una absorción de energía superior para las zoea (I, II y III) que mysis (I, II y III), esto probablemente a una interacción entre las tasas de respiración y de excreción provocada por variaciones en la eficiencia de absorción de las larvas. Los pesos obtenidos en ambas larvas no resultaron con diferencias entre las dietas suministradas.
Palabras clave: Amonio, brachionus plicatilis, oxígeno, camarón blanco (Fuente: MeSH).
INTRODUCTION
In Mexico, shrimp culture has been based solely on post larvae of Pacific white shrimp Litopenaeus vannamei produced under commercial laboratory conditions. Penaeid shrimp in laboratory conditions are adequately fed during larvae development both in quality and quantity in order to keep the larvae alive and ensure morphologic changes in larvae substrates from nauplius to post larvae (1).
Presently, commercial diets are available that constitute alternatives to live diet based on microalgae (2,3) where these diets play a fundamental role as feeding complements for zoea larvae of L. vannamei. However, due to characteristics such as nutritional composition, digestibility, presentation, floatability and above all ease of ingestion, they have not yet been optimized for the constant demand created by larva cultivators (4). Additionally, satisfactory results have not been obtained and therefore the producers continue to require live feed (micro algae) at least during the first stages of larvae development of L. vannamei white shrimp (5).
Due to the above and the uncertain market in the demand of C. muelleri cysts to obtain nauplius feed for mysis larvae, production laboratories could eventually not be able to meet the demand for post larvae penaeid shrimp, which could lead to a serious problem for the shrimp sector as it strives to provide larvae feed. An urgent and necessary alternative is to use diets for shrimp larvae based on microorganisms that can substitute Artemia nauplius such as copepods and (6) rotifers (7,8).Rotifers are an important feeding source for shrimp larvae since they have high dietary value (9). Production techniques for mass cultivation of brachionus plicatilis and Brachionus rotundiformis rotifers has been described, which makes these microorganisms probable alternatives to feeding for the first stages of the life cycle of penaeid shrimp, in particular during the mysis stage (8,9), as well as fish alevin (10). However, for rotifers, larvae or microscopic water species there are hydrologic factors that are of interest, such as temperature, pH, salinity and dissolved oxygen, among others, that can alter physiological processes such as respiration and ammoniacal excretion (11) in those aquatic organisms.
However, it is not only necessary to propose new alternative diets that can substitute at some point the Artemia nauplius for rotifer, but also what implications this has in larvae and how this affects these microorganisms in physiological processes such as, among others, respiration and ammoniacal excretion in L. vannamei Larvae.
Studies exist that analyzed the oxygen consumption and ammoniacal excretion in white shrimp post larvae using feed that is not alive (11,12). However, in L. vannamei larvae investigations have not been done on the effect that traditional feed (microalgae and Artemia nauplius) and non-traditional feed (microalgae and B. plicatilis rotifer) have on respiration and excretion of zoea and mysis larvae in white shrimp. Therefore, the objective was to analyze the consumption of oxygen and ammonia excretion in zoea and mysis larvae of L. vannamei white shrimp fed with different diets (microalgae and Artemia nauplius and microalgae and rotifer) under laboratory conditions.
MATERIALS AND METHODS
Support cultures. The species used as support cultures, such as microalgae, rotifer and C. muelleri, were Chaetoceros muelleri, brachionus plicatilis y Artemia franciscana, respectively. The strains of microalgae were provided by the collection from the Centro de Investigaciones Científicas y de Estudios Superiores de Ensenada (CICESE), rotifer was provided by the Centro de Investigaciones Biológicas del Noroeste (CIBNOR) and Artemia cysts were acquired from a commercial business. The techniques used to cultivate the microalgae (13), rotifer (8) and to decapsulate the Artemia cysts and obtain nauplius (14) followed what was proposed by those authors. The larvae correspond to one lot of L. vannamei in nauplius III and were provided by a commercial laboratory in the south of Sinaloa, Mexico. The larvae were transported for a period of 2 h in a container in plastic bags with seawater (35ups) filtered, oxygenated to saturation, and between 26 and 27°C.
Experiment design. In the laboratory larvae were acclimatized (1°C/h) to the experiment temperature and salinity (29±0.2°C and 35ups) and were placed in a 250 L recipient with filtered seawater to 1 µm and treated with activated carbon. They were constantly aerated with bubbles and the air was filtered to 1 µm. The larvae was maintained with daily water changes of 25 to 30% and bio deposits were eliminated by a syphon protected with a Nytex screen of 200 µm.
When the larvae were in zoea I to mysis III stages they were concentrated in a submerged sieve and were later placed in four recipients of 15 L (aquarium) to a density of 100 larvae/L (1500 larvae/aquarium). Afterwards the concentration of the feed was verified. Initially, 50 larvae were obtained per phase and the dry unit weight (PSU) and the organic unit weight (POU) of the larvae were estimated. Additionally, the PSU and PSO per individual was estimated for the microalgae, rotifer and C. muelleri nauplius; these groups were concentrated separately in 25 mm Whatman GF/C fiberglass filters, previously calibrated. The PSU and PSO of the microalgae was expressed in pg/cel-1 (pg=picograms). In the aquariums, the elimination of the excess feed and feces were extracted by a syphon protected by a 250 µm nylon NITEX meshing to impede the exit of zoea larvae and 600-700 µm for mysis.
The estimation of oxygen consumption and ammonium excretion was determined separately in zoea and mysis larvae. In zoea, a total of 102 300 ml BOD bottles were used, of which, 32 bottles corresponded to each sub phase (Zoea I, II y III) with 60 larvae (closed respirometers), whose sole feed was microalgae (C. muelleri), and six bottles were considered control (no larvae).
In mysis, a total of 204 300 ml BOD bottles were used, corresponding to 64 bottles for each sub phase (mysis I, II and III) with 60 larvae (closed respirometers), and four bottles were considered control. Of these 64 bottles, 32 were fed with microalgae -C. muelleri- and C. muelleri nauplius (ma+nau) and 32 bottles with microalgae diet -C. muelleri- and rotifer (ma+rot). All the bottles with both larvae phases were kept in a rectangular fiberglass recipient (124x60x19 cm) with water recirculated with a LITTLE GIANT pump submerged to 1/16 Hp and a constant temperature of 29°C with a heater and FINNER HC-0800 thermoregulator.
Determining the oxygen consumption and ammoniacal excretion for each bottle of the zoea and mysis larvae was recorded four hours after beginning the experimental phase. The concentration of oxygen was directly measured in each bottle with an oximeter and an ORION polarographic electrode. To estimate the consumption of oxygen for each control, the differences in the average of oxygen in the control bottle (without larvae) was subtracted from the average amount of oxygen in the bottles with larvae, thus obtaining the average amount of oxygen consumed by the larvae. To estimate the consumption per larva, the amount of oxygen consumed in each bottle was divided among the number of larva (60 larvae) contained in each bottle, thus obtaining the oxygen consumption per larva (12,15,16), the oxygen consumed was expressed as µg O2/larva-1/h-1. Later, the oxygen consumption per larva was converted into units of energy under the supposition that 1 mg of O2 consumed is equivalent to 14.06 J (17), the consumption of oxygen in energy per larva was expressed as mJ/larva-1/h-1.
Simultaneously with the oxygen readings for each bottle, ammonium was determined using the indophenol blue method (18), which consisted of obtaining 10 ml of water in each bottle (with and without larvae) which were deposited in duly labeled test tubes. To each was added 0.4 ml of phenol alcohol solution, 0.4 ml of nitroprusside and 1 ml of oxidizing solution (sodium citrate and sodium hypochlorite), after which the tubes were shaken and left to rest for two hours to develop the color of the chromophore mix, after which absorbency readings were taken in a HACH 4000UV-VIS spectrophotometer with a wave length of 640 nm. To estimate the excretion per larva, it was calculated the same way as oxygen consumption. The ammonium excretion was expressed as µg N-NH4+/lar-1/h-1. The amount of ammonium excreted by larva was converted into energy units with the idea that 1 mg of nitrogen ammonium (N-NH3) is equivalent to 24.87 J (19), the excretion of ammonium in energy was expressed as mJ/lar-1/h-1.
The O:N index (rate of oxygen consumption and ammonium excretion) was estimated based on the values of oxygen consumption and ammonium excretion of the larvae kept in respirometers during the experimental phase. The physiological rates (consumption and excretion) of the larvae for both components were transformed into atoms/grams to estimate the O:N using the supposition of respiratory thermochemistry (17). This index was used to estimate the energy component of proteins, lipids and carbohydrates for L. vannamei larvae. It was considered that values between 3 and 16 obtained from this index indicated protein oxidation, from 50 to 60 indicated catabolism of proteins and lipids, and values above 61 corresponded to carbohydrates (20).
Statistical analysis. Using the data of oxygen consumption and ammonium excretion in zoea and mysis substages, normality (Lillieford) and homoscedasticity (Bartlett) tests were performed, and depending on the results, in zoea a one way ANAVA was applied, while mysis corresponded to a two factor ANAVA (substage and diet). In cases where the results were significant, multiple comparison tests were performed (Tukey -parametric- or Tukey Type -non parametric). In all cases, a level of significance of 0.05 (21) was used, and measurement adjustments were calculated using the Statistica V7.0 packet (22).
RESULTS
Total and organic dry weight. The total and organic dry weight of larvae increased from zoea I by 11.3 (PSU) and 8.7 µg (POU) to 92.1 (PSU) and 71.7 µg (POU) for the PL1 phase. Weight increase for the organisms between the substages showed different growth rates. In mysis I the increase in total dry weight fluctuated from 47 to 74%. However, the inorganic material of the larvae did not behave according to larva development, with percentages between 17.4 and 25% (Table 1).
Respiration. The oxygen consumption rates in Zoea increased progressively from 1.47 in Zoea I to 2.15 µg O2/larva-1/h-1 in Zoea III. The equivalent energy use per hour in the larvae varied from 20.6 to 30.2 mJ/lar-1/h-1. When the oxygen consumption data were analyzed statistically, these showed significant differences between Zoea I and II, but not between II and III (Table 2).
In mysis fed with microalgae and C. muelleri nauplius, oxygen consumption was different in the substages, with a minimum and maximum variation of 1.9 and 2.4 µg/O2/lar-1/h-1, which corresponded to an energy output of 27.9 and 34.8 mJ/lar-1/h-1, respectively. While the mysis larvae that were fed with the alternative diet (microalgae and rotifers) did not show differences between the three substages, with consumption between 2.2 and 2.4 µg/O2/lar-1/h-1, its equivalent in energy consumption varied from 32.0 to 33.8 mJ/lar-1/h-1. Additionally, statistically there were no significant differences observed in oxygen consumption measurement in mysis substages (Table 3).
Lower energetic use was seen in mysis I fed with Artemia nauplius, since they possibly consumed less feed; from mysis II no differences were seen between the substages (2.3 to 2.4 µg/O2/lar-1/h-1), and diets (33.5 to 34.8 mJ/lar-1/h-1), although the values were intermediate during mysis I fed with microalgae and B. plicatilis rotifers. As was seen with the oxygen consumption in mysis, metabolic output showed no statistical differences between the diets given to those larvae (Table 3).
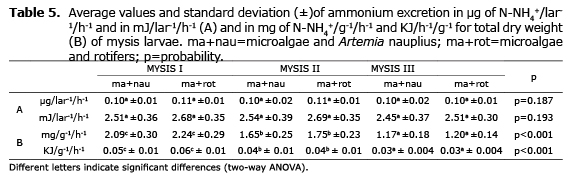
Ammonium excretion. In zoea, the excretion of NH4+ in energy units was inferior to those estimated for respiratory activity, and did not present a gradient related to the weight and development time of larvae. The individual excretion rate was around 0.06 µg N-NH4+/lar-1/h-1, corresponding to 5 and 7.5% of the metabolic use recorded during respiration. The normalized production rate of NH4+ per unit of weight diminished progressively, presenting significant differences in the zoea substages where the metabolic cost in zoea I respiration was 7% and between zoea II and III was 5%. The values of zoea excretion resulted statistically significant in larvae development, and the excretion values increased with the substage, although no statistical differences were detected between the diets fed in such larvae (Table 4).
In mysis, the excretion rate did not show differences between the diets fed to the larvae, and the same can be said for individual weight and in regard to the developmental phase. On average, values from 0.10 to 0.11 µg N-NH4+/lar-1/h-1, and from 2.5 to 2.6 mJ/lar-1/h-1 were obtained, which correspond to 8 and 10% of the metabolic use calculated from the respiration rate for larvae (Table 5).
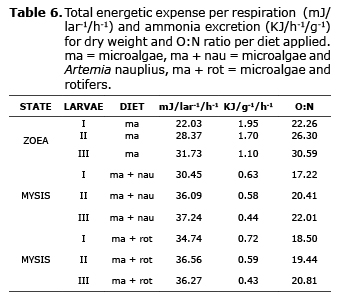
Metabolic expense and O:N ratio. The average respiration rates and ammonium excretion, which were mainly produced by catabolism of the protein, aided in estimating the metabolic expense per substage, which was between 22 (zoea I) and 31.7 mJ/lar-1/h-1 (zoea III), which corresponded to a daily energetic expense of 0.53 and 0.76 mJ/lar-1/day-1, respectively.
The values of metabolic expense normalized per unit of total dry weight progressively diminished, obtaining total daily energetic expenses of 46.8 (zoea I), 40.8 (zoea II) and 26.4 kJ/día-1/g-1 (zoea III) of dry weight of larvae, which indicated a minimum reduction between zoea I and II with respect to zoea III. Although the total daily metabolic expense for the zoea III stage represented approximately 56% with regards to the metabolic expense of zoea I.
In mysis, the individual metabolic expense with microalgae and C. muelleri nauplius feedings showed a minimum of 30.5 mJ/lar-1/h-1 and a maximum of 37.2 mJ/lar-1/h-1, while when based on microalgae and rotifers, the variation was 34.7 and 36.6 mJ/lar-1/h-1. However, energetic expense values were very similar between substages, independently of the diet fed to the larvae. Conversely, energetic expense per unit of weight diminished by approximately 60% between zoea III and mysis I, with minimum values progressing according to larval development and unrelated to the type of feed given to the larvae.
The O:N ratio in zoea larvae increased parallel to larval development, with values from 22.3 to 30.6. Mysis had lesser O:N ratio values than zoea, between 17.2 and 22, which determined that the proteins in both stages were used as metabolic substrate during larval development of L vannamei (Table 6).
DISCUSSION
The final weights reached in the postlarva stage (PL1) obtained in development were considerably superior in the same species and age than those obtained by other authors in laboratory conditions (8). The probable difference in weights obtained could be related to environmental characteristics in which the larvae developed between nauplius I and II. Although they were part of the same production lot, the weight of the larvae could have been influenced by a genetic variability in the nauplius used. It we take into account that larval development is directly related to water conditions and feed, then the results of this study with respect to average weights between the diets could be considered as indicators of adequate conditions in which the laboratory tests were performed.
Also, the same water conditions were maintained among all the bottles, and only the type of feed given to the larvae was changed, so that weight variations obtained could be attributed to different nutritional components in diets based on microalgae and C. muelleri nauplius or microalgae and rotifers, in order to achieve morphologic changes between mysis and postlarva stages, which is necessary so that larvae can reach said phase according to changes in the feeding habits during development. In general, satisfactory results have been reported regarding survival and metamorphosis when diatom microalgae have been used as feed for white shrimp larvae (2,3).
The respiration rate in crustaceans and in particular L. stylirostris and L. vannamei larvae can be modified by external factors such as salinity, light intensity, dissolved oxygen and temperature, as some authors have mentioned (23), although in this study those factors were controlled. Oxygen consumption values obtained in mysis larvae fed with microalgae and C. muelleri nauplius or with the alternative diet (microalgae and rotifers) coincide with oxygen consumption of larvae fed Litopenaeus setiferus (24), who indicated that oxygen consumption varies according to the type of feed. These authors, as in this study, determined greater oxygen consumption when the larvae were fed with a concentration of one or two C. muelleri nauplius/ mL-1. Additionally, oxygen consumption in mysis II and III were similar when 0.5 nauplius/mL-1 were provided, but when the larvae were fed a concentration of 0.1 nauplius/mL-1, oxygen consumption was minimum.
The results obtained in oxygen consumption did not coincide with that reported in L. vannamei larvae (25), who showed that oxygen consumption diminished with larval development, that is, as morphological changes in the larvae from zoea to mysis took place, respiration decreased. However, those authors indicated that the consumption of oxygen did not show significant differences between the stages. In this study, larvae fed with microalgae and C. muelleri nauplius increased oxygen consumption in developing between the three mysis phases (I, II and III), but when they were statistically analyzed, no differences in oxygen consumption were detected between mysis larvae II and III.
Metabolic expenses in this study of Litopenaeus setiferus larvae (26) were superior to the amount of energy used in respiration when compared with energy used in growth, with the exception of the zoea III stage. For example, those authors indicated that the larvae assimilated 3.59 J, of which approximately 42% was used in growth and 52% of the energy was used for respiration, which coincides with that reported on larvae for Pacific white shrimp L. vannamei (6) and Penaeus setiferus from the Gulf of Mexico (24).
Higher energy absorption was seen in zoea larvae (I, II, and III) than in mysis (I, II, and III), probably due to an interaction between respiration and excretion rates from variations in absorption efficiency as a consequence of variations in feed availability and the delay in the digestive tract of the larvae. However, differences observed in the present study in evaluating the metabolic expense measured as respiration and excretion are due to the fact that the larvae were previously fed, which included three main components of respiration and excretion (routine, standard and post-feeding), where the last one could alter the physiologic variation according to the type and amount of feed ingested by the larvae.
In conclusion, the zoea larvae required greater energy absorption than mysis as a consequence of the interaction between respiration and excretion. Also, the mysis larvae fed with rotifers instead of C. muelleri nauplius could be less efficient, since B. plicatilis is not considered to be adequate feed during the mysis II and III phase, mainly due to size, making it difficult for larvae to capture, and therefore requires greater energetic expense. However, the weight obtained for zoea and mysis larvae for L. vannamei shrimp showed no differences according to the diets provided.
Acknowledgements
To the CONACYT 38232-B project for the resources granted. To the staff at Cuerpo Académico Consolidado UAS-CA-162. To the Programa de Posgrado Regional para el Doctorado en Biotecnología of the Universidad Autónoma de Sinaloa.
REFERENCES
1. Arzola GJ, Piña VP, Nieves SM, Medina JA. Supervivencia de postlarvas de camarón blanco Litopenaeus vannamei a diferentes salinidades y temperaturas. Rev MVZ Córdoba 2013; 18(2):3618-3625. [ Links ]
2. Piña VP, Nieves M, Ramos L, Chavira CO, Voltolina D. Survival, growth and feeding efficiency of Litopenaeus vannamei protozoea larvae, fed different rations of diatom Chaetoceros muelleri. Aquacult 2005; 249(1-4):431-437. [ Links ]
3. Piña VP, Voltolina D, Nieves M, Robles M. Survival, development and growth of the pacific white shrimp Litopenaeus vannamei protozoea larvae, fed with monoalgal and mixed diets. Aquacult 2006; 253(1-4):523-530. [ Links ]
4. Páez, OF. Retos y perspectivas de la camaronicultura en la zona costera. Rev Lat Rec Nat 2005; 1(1):21-31. [ Links ]
5. Piña VP, Medina JA, Nieves M, Leal S, López-Elías J, Guerreo MA. Cultico de cuatro especies de microalgas con diferentes fertilizantes utilizados en acuicultura. Rev Invest Mar 2007; 28(3):225-236. [ Links ]
6. Isiordia PE, Puello A, D'Abramo L, González H. Evaluación de la actividad enzimática y contenido de proteína en larvas de camarón blanco Litopenaeus vannamei alimentadas con diferentes dietas. Redvet 2006; 7(4):1-13. [ Links ]
7. Rojo CA, Román RJ, Rodríguez MG, Nieves SM, Piña VP, Medina JA. Balance energético del rotífero Brachionus rotundiformis alimentado con cuatros especies de microalgas. Univ Cienc 2012; 28(3):231-244. [ Links ]
8. Piña VP, Nieves SM, Voltolina D, Chavira C. Crecimiento, desarrollo y supervivencia de mysis de (Litopenaeus vannamei) alimentadas con nauplios de Artemia y con el rotífero brachionus plicatilis. Rev Invest Mar 2004; 25(3):245-251. [ Links ]
9. Planas M, Vázquez JA, Marqués J, Pérez-Lomba R, González MP, Murado M. Enhancement of rotifer brachionus plicatilis growth by using terrestrial lactic acid bacteria. Aquacult 2004; 240(1-4):313-329. [ Links ]
10. Prieto M, Castaño F, Sierra J, Logato P, Bolero J. Alimento vivo en la larvicultura de peces marinos: copépodos y mesocosmos. Rev MVZ Córdoba 2006; 11(1):30-36. [ Links ]
11. Arzola GJ, Flores LF, Izabal A, Gutiérrez Y. Crecimiento de camarón blanco Litopenaeus vannamei en un estanque rústico a baja salinidad. Aquatic 2008; 28(1):8-15. [ Links ]
12. Spanopoulus HM, Martínez CA, Vanegas RC, Rosas C, Ross LG. The combined effects of salinity and temperature on the oxygen consumption of juvenile shrimps Litopenaeus stylirostris. Aquacult 2005; 244(1-4):341-348. [ Links ]
13. Nieves M, López D, Medina MA, Piña P, Leal S, López EJ. Producción y calidad de Chaetoceros muelleri a diferentes concentraciones de nutrientes y densidades de inóculos. Rev Invest Mar 2009; 30(2):123-133. [ Links ]
14. Gelabert R, Brito R, Gaxiola MG, Castro T, Rosas C. Efecto de nauplios de Artemia franciscana enriquecidos sobre el crecimiento, supervivencia y resistencia al estrés de postlarvas (PL5-40) de Litopenaeus vannamei. Univ Cien 2008; 24(1):29-40. [ Links ]
15. Re AA, Díaz HF. Effect of different oxygen concentrations on physiological energetic of blue shrimp Litopenaeus stylirostris. O Zool J 2011; 4(1):1-8. [ Links ]
16. Valenzuela QW, Rodríguez QG, Ponce PP, Esparza LH. Efecto de diferentes combinaciones de temperatura y salinidad sobre el consumo específico de oxígeno en el camarón blanco Litopenaeus vannamei. Rev Biol Mar Ocean 2011; 46(3):303-311. [ Links ]
17. Gnaiger E, Forstner H. (Eds) Polarographic oxygen sensors. Berlin. 1983. [ Links ]
18. Rodier J. Análisis de las aguas: naturales, residuales y agua de mar. Ed. Omega, Barcelona. 1981. [ Links ]
19. Elliot JM, Davison W. Energy equivalents of oxygen consumption in animal energetic. Oecología 1975; 19(1):195-201. [ Links ]
20. Mayzaud P, Conover RJ. O:N atomic ratio as a tool to describe zooplankton metabolism. Mar Ecol Prog Ser 1988; 45(1):289-302. [ Links ]
21. Zar JH. Biostatistical analysis. Upper Saddle River, USA: Prentice-Hall Inc; 2009. [ Links ]
22. StatSoft Inc. Statistica for Window version 7.0. Tulsa, Oklahoma, USA: StatSoft Inc; 2004. [ Links ]
23. Re AD, Díaz F, Sierra E, Gómez-Jiménez S. Oxygen consumption, ammonium excretion and osmoregulatory capacity of Litopenaeus stylirostris exposed to different combinations of temperature and salinity. Cienc Mar 2004; 30(3):443-453. [ Links ]
24. Brito R, Chimal ME, Gelabert R, Gaxiola G, Rosas C. Effect of artificial and natural diets on energy allocation in Litopenaues setiferus and Litopenaeus vannamei early postlarvae. Aquacult 2004; 237(4):517-535. [ Links ]
25. Gallardo P, Martínez G, Brito A, Barrera J, Pedroza-Islas R, Cuzon G, et al Effect of Artemia nauplii replacement by an artificial feed containing kril hydrolysate on ingestion rate, oxygen consumption and energy budget in the mysis of Litopenaeus vannamei. Nauplius 2003; 11(2):69-81. [ Links ]
26. Lemos D, Phan VN, Álvarez G. Growth, oxygen consumption, ammonia-N excretion, biochemical composition and energy content of Litopenaeus setiferus (Crustacea: Decapoda: Penaeidae) early postlarvae in different salinities. J Exp Mar Biol Ecol 2001; 261(1):55-74. [ Links ]