INTRODUCTION
Phototoxic dermatitis (DFS), also known as hepatogenic or secondary photosensitization, is defined as skin sensitivity to sunlight due to the presence of photodynamic agents 1 in severe cases of photosensitive dermatitis. It is known in Brazil as "requeima" 2 and in Colombia it is known on the Atlantic coast as "fiebre candela" and in the Andean region as "Brasa, lamparón or fiebrón" 3. It has been reported in different species; however, the largest presentation of casuistry has been reported in ruminants, including reports for sheep 4-6, goats 7, buffalo 8,9 and bovines 2,10. Also, greater susceptibility is reported in sheep than in cattle 7, and it mostly affects young animals rather than in adults 11.
The presence of photodynamic substances produced by higher plants that are reactive to light can lead to significant damage to sensitive tissues and can cause irritation, abrasion and secondary skin infections 12. DFS occurs when hepatotoxic substances, whether chemical factors (phenothiazine, tetracycline or sulfonamide) or biological factors (toxic plants or infectious agents like the fungus Phytomices chartarum) cause sufficient damage the liver to prevent excretion of photoactive phytoporphyrins 13.
An important photodynamic agent is phylloerythrin, produced by chlorophyll decomposition, which accumulates in significant concentrations in the blood flow of affected animals, thereby inducing photosensitivity. Phylloerythrin occurs as a normal byproduct of rumen function, when large amounts of chlorophyll are decomposed by bacteria in the gastrointestinal tract and are absorbed through the rumen into the bloodstream where they are removed by conjugation and excretion in bile 12. When they are not eliminated by an injured liver, phylloerythrin concentrations increase in blood circulation and then accumulate in the skin. Since this is a photodynamic agent, it absorbs certain ultraviolet wavelengths (UV), becomes active, and transmits this extra energy to the circumjacent cells, which results in cell injury 14.
Scheie et al 15, in studies on sheep, established normal phylloerythrin plasma concentrations at less than 0.01 mM/L, and estimated values superior to 0.03 mM/L as sufficient for photosensitization to take place in affected animals. Although how phylloerythrin acts as a photodynamic agent is unknown, studies have shown that it binds to Golgi and mitochondria, indicating that these organelles may be the main target of this phototoxic compound 12.
Over the years, the toxic power of Brachiaria spp. was added to the concomitant action of the fungus Phytomices chartarum, which synthesizes and releases esporodesmin through its spores, which causes liver injury. This mycotoxin belongs to the class of epipolythiodioxypiperazine toxic compounds and is considered highly hepatotoxic and causes in the host a hepatocyte immune response mediated by foamy macrophages, which when swallowed form "digestion chambers" that occlude bile ducts and impede phylloerythrin excretion with subsequent photosensitization. However, studies on photosensitive animals grazing in paddocks with Brachiaria showed very low amounts of P. chartarum fungal spores and with no sporidesmin production, so it was concluded that such cases were not due to the presence of the fungus, but to toxic conditions of Brachiaria components 16.
Quinn et al 12 showed that secondary skin photosensitization due to liver impairment could be influenced by the presence of lithogenic steroidal saponin present in Brachiaria spp. pastures. Wina et al 17 explain that although saponin is not essential to the life of the plant, it ensures survival in the ecosystem, located in the most vulnerable parts of the plant to fungal and bacterial attack or predator insects, and are related to the defense mechanism of the plant by acting as a protective chemical barrier.
A large number of hepatotoxins derived from plants have been reported, including steroid saponins, terpenes and tannins which can also cause death through acute liver failure. However, if they are ingested in smaller doses that the animal survives, subsequent liver alterations may result in secondary photosensitization. Thus, Riet-Correa et al 7 reported that Brachiaria spp. pastures have varying degrees of toxicity associated with saponin levels, indicating that protodioscin (saponins in Brachiaria decumbens), that when hydrolyzed form sapogenin, diosgenin and yamogenin. When metabolized in the digestive tract, epismilagenin and episarsasapogenin sapogenins are formed that combine with glycoronic acid, forming glycuronides that bind calcium ions and form insoluble salts that are deposited as crystals. Oliveira et al 9, Faccin et al 14, and Santos et al 18 demonstrated that these lithogenic steroidal saponins present in the Brachiarias spp. induce migration of foamy macrophages and stimulate the formation of crystals in hepatocytes and bile ducts, obstructing bile canaliculi with the consequent accumulation of phylloerythrin in circulation and in the skin, which causes phototoxic dermatitis.
Clinical manifestations begin with loss of appetite, excitability, pruritus, bilateral epiphora, cutaneous edema on ears, chin, flanks, and cauda and inguinal folds. In most cases the animal manifests photophobia 5,13, and as the disease progresses this becomes more pronounced and is considered a sign of photosensitivity. Mucous membranes become jaundiced, the animal becomes dehydrated, with progressive emaciation due to loss of appetite that increases and even reaches cachectic state, pronounced skin wrinkling especially around the ears and perineal and costal regions 13,18. Injuries are primarily in areas most exposed to the sun, especially where there is less pigmentation or lower fur density such as eyelids, ears, perineum and dorsal and erythema and edema begin, and later the formation of vesicles, exudation, necrosis and desquamation 7.
It is important to use blood tests to evaluate the catalytic activity of liver enzymes, particularly GGT (Gamma Glutamyl Transferase) and AST (Aspartate Amino transaminase), since a significant increase in serum is indicative of hepatic impairment, especially GGT, which is located at the canaliculi hepatic level 4-6.
In histopathology, hematoxylin eosin coloring in the affected skin can be observed epithelial degeneration with vacuolar degeneration, chronic necrotizing dermatitis and necrosis in the epidermal layer and edema in the dermal as well as macrophage infiltration and fibrinoid degeneration in the middle arteries 4. Focal chronic periportal hepatitis can be found, with numbness and retention in hepatocytes and bile canaliculi, as well as increased neutrophils in the liver parenchyma, presence of macrophages with negative cytoplasmic images of acutiforme crystals and foamy cytoplasm in the interior of the sinusoids and periportal region, respectively. Similarly, bile duct hyperplasia can be observed and the presence of crystalloid birefringent that occludes bile ducts 5,9.
Treatment consists of immediate removal of the animals from pastures with Brachiaria spp. and sunny areas, and applying liver protectors, anti-histamines and moisturizing solutions as well as using antiseptics and healing ointments on the worst lesions. Another alternative is the use of dexamethasone acetate and vitamin B complex, and both treatments may have favorable results depending on the exposure time and extent of liver damage 19.
The aim of this study was to conduct a study on the clinical and histopathological aspects of DFS in twelve Zebu calves department of Cordoba, Colombia.
MATERIALS AND METHODS
Type of study. A descriptive type study was done, non-probalistic in animals with cutaneous lesions compatible with DFS.
Location. It was conducted between February and March in the department of Córdoba, Colombia, located between coordinates 7°23' and 9º26' NL and 74°52' and 76º32' WL of Greenwich, at a height of 30 m with average annual temperature of 28°C, relative humidity 82%, annual rainfall 1400 mm in a rainforest climate 20.
Study animals. Twelve male Cebu calves with an average age of 12 to 18 months were used. All showed signs of photophobia, dehydration, progressive emaciation, jaundiced mucous membranes and skin lesions such as cracked skin with leathery appearance around the ears, perineal, epigastric and costal regions and peeling skin with some scabbing, symptoms compatible with DFS.
Methodology. All animals underwent clinical evaluation and histopathological characterization of skin lesions, for which high definition photographs for further analysis (DSC-HX10V Sony, China) were taken. Similarly, after sedation (acepromazine 1%, Zoo, Colombia) and application of local anesthesia (Lidocaine 2%, Synthesis, Colombia), excisional biopsy of tissue from the periphery of the skin lesion was performed and fixed in 10% formalin (v/v) 9 and taken to the Pathology laboratory of the department of Animal Sciences at the University of Cordoba, Colombia, where they were processed to inclusion in paraffin. Subsequently, they were transferred to the laboratory of Structural Biology in the department of General Biology at the Federal University of Viçosa, Brazil, where they were processed for routine histopathologic evaluation, cut to 5μm on a microtome (Leica RM2125 RTS, Japan) and stained with hematoxylin-eosin (HE), Gomori trichrome (T-G) and Picrosirius Red/Polarization (PR/P). For microscopic photo analysis of the samples, a microscope with polarization options (Olympus BX-53(r), Brazil) was used from the Molecular Systematics Laboratory/BEAGLE of the Animal Biology Department of the Federal University of Viçosa, Brazil.
10 ml blood samples were collected without anticoagulant by venipuncture of the jugular using a vacuum collection system to obtain serum from the twelve animals with DFS and six other clinically healthy animals from the lot as the sick animals to perform biochemical tests for liver and kidney function. Each sample was labeled and stored in refrigeration at 4°C (Plastic Refrigerator, Imusa, Colombia) to be later taken to the "Cereté" Clinical Laboratory in the municipality of Cereté, Córdoba, where GGT and AST serum levels were determined to evaluate liver function as well as urea and creatinine to assess renal function 14.
After clinical evaluation, injury characterization, and sampling, a therapeutic strategy was instituted consisting of suspending the gentian violet treatment that had been applied which potentiates sunlight, as well as immediately removing the animal from the Brachiaria decumbens paddock to one with Dychanthium aristatum grass and shade provided by trees and shade structures. Likewise, a corticoid-based dexamethasone was applied at a dose of 0.1 mg/kg intramuscularly and 10 centimeters of a commercial B complex multivitamin intramuscularly for 5 days, as well as administering a hydrating solution consisting of a mixture of molasses with sodium bicarbonate.
Ethical aspects. The animals were not subjected to pain an or unnecessary stress, and were immobilized taking into account the technical standards in handling and restraining animals in compliance with the Universal Declaration of Animal Rights concerning international biomedical research with animals CIOMS (Council for international Organizations of Medical Sciences) established by UNESCO (United Nations Educational, Scientific and Cultural Organization) and WHO (World Health Organization) 1949 and the Law 84 from October 27, 1989 (Colombian Animal Protection Statute) 21.
Statistical analysis. The data were tabulated and analyzed descriptively, and the statistical differences in the results of liver and kidney function tests of DFS animals with clinically healthy ones and the chi-square test was used, using S.A.S Software 9.1.3.
RESULTS
The observed clinical manifestations, anatomopathological characteristics of the lesions, and the results of histopathological and serological tests confirmed the DFS diagnosis in the 12 cattle studied according to what is reported as a diagnostic method for DFS in ruminants 2,9,10,12,14. All calves had lesions on ears, chin, neck, chest and epigastric, perineal and inguinal regions, and all had mucosa with some degree of jaundice, dehydration and emaciation (Figure 1).
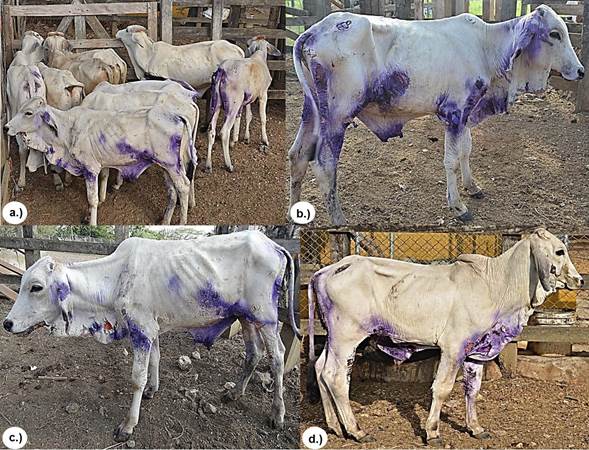
Figure 1 Group of animals with DFS. a.), b.) and c.) Skin lesions on ears, chin, neck, thorax, and epigastric, perineal and inguinal regions, all with a loss of health.
Clinical manifestations of skin lesions were characterized by the presence of erythema, edema, cracked skin with a leathery appearance and skin peeling in some areas with scabbing (Figure 2).
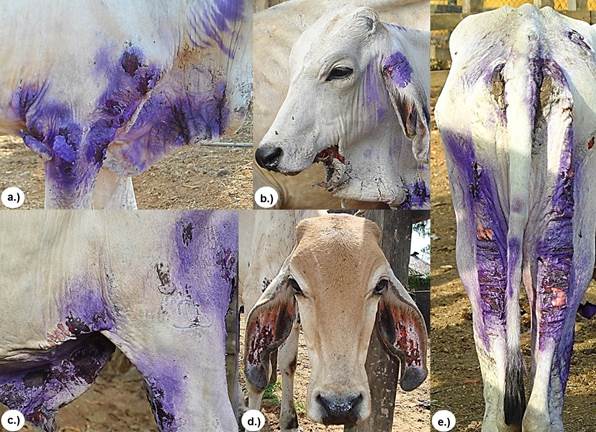
Figure 2 Clinical manifestations of cutaneous lesions characterized by the presence of erythema, edema, cracked skin with a shriveled appearance and skin peeling in some areas with scabbing at a.) shoulder and elbow, b.) dewlap c.) inguinal region and foreskin, d.) ears and e.) perineal and posterior region of pelvic areas.
Table 1 shows the results of liver function tests (GGT and ALT) and renal function (urea and creatinine), of which only the hepatic enzymes of animals with DF showed considerably elevated catalytic activity, with statistically significant differences (p<0.05) with respect to the catalytic activity of liver enzymes in the group of clinically healthy animals. Urea and creatinine concentrations in blood were within normal parameters for the group with DFS and the clinically healthy group taken as control.
Table 1 Serum values of GGT, AST, urea and creatinine in a group of calves with DFS and the clinically healthy group in that grazed on Brachiaria decumbens in the Department of Córdoba, Colombia..
| Variable (*) | GGT (U/L) (6,1-17,4) | AST (U/L) (78-132) | UREA (mg/dL) (23-58) | CREATININE (mg/dL) (1-2) | |||
|---|---|---|---|---|---|---|---|
| ANIMALS | |||||||
| DFS | 1 | 275.6 | 315.0 | 73.0 | 2.3 | ||
| 2 | 129.3 | 230.0 | 38.0 | 0.95 | |||
| 3 | 117.0 | 223.0 | 34.0 | 1.2 | |||
| 4 | 294.0 | 81.2 | 38.5 | 1.8 | |||
| 5 | 158.0 | 33.9 | 34.2 | 0.8 | |||
| 6 | 174.0 | 35.0 | 35.8 | 1.35 | |||
| 7 | 116.6 | 232.0 | 33.0 | 0.91 | |||
| 8 | 170.4 | 210.0 | 28.0 | 1.3 | |||
| 9 | 111.3 | 190.0 | 48.6 | 1.7 | |||
| 10 | 140.5 | 215.0 | 42.6 | 1.9 | |||
| 11 | 180.3 | 250.0 | 32.0 | 1.6 | |||
| 12 | 132.6 | 235.0 | 35.0 | 1.5 | |||
| Mean ± D.E | 166.6 ±60.1 | 187.5 ±88.9 | 39.4 ±11.8 | 1.4 ±0.4 | |||
| HEALTHY | 1 | 17.0 | 38.3 | 40.0 | 0.9 | ||
| 2 | 21.3 | 45.2 | 32.0 | 1.8 | |||
| 3 | 23.3 | 35.3 | 46.0 | 2.0 | |||
| 4 | 14.0 | 40.1 | 27.0 | 0.8 | |||
| 5 | 12.0 | 23.7 | 21.0 | 0.7 | |||
| 6 | 18.6 | 22.8 | 20.0 | 1.9 | |||
| Mean ± D.E | 17.7 ±4.3 | 34.2 | ±9.1 | 31.0 ±10.4 | 1.4 ±0.6 | ||
* Reference values according to Oliveira et al (9).
In all biopsies of skin tissue stained with the H&E stain, similar histopathologic characteristics were observed that consisted of extensive clotting necrosis in the epidermal layer containing cellular debris and basophilic material resembling bacterial colonies, a large number of inflammatory mononuclear cells, macrophages and neutrophils, as well as epithelial degeneration with hyperkeratosis, disorganization of fibroblasts and fibrous connective tissue (Figure 3). In the T&G stain, poor dermal proliferation of disorganized fibroblasts, low presence of diffuse connective tissue, and disorganized collagen fibers (Figure 4) were observed and in the P-R/P stain areas of birefringence were observed, indicating a moderate presence of mature type I collagen (bright red in polarization) and type III (bright green in polarization), (Figure 5).
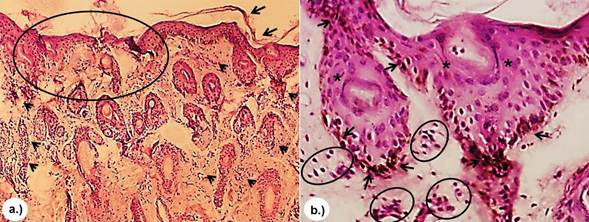
Figure 3 H&E stain. a.) Presence of a large amount of neutrophilic PMN inflammatory cells (arrow) as well as marked epithelial degeneration (circle) with hyperkeratosis (arrow) 4x. b.) Presence of acantholytic cells in the granular layer (arrow), neutrophilic PMN (circle) and acantholysis (asterisk) 10x.
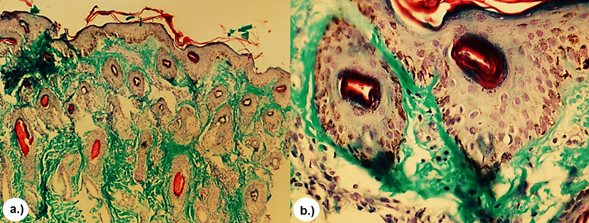
Figure 4 T&G stain. Moderate dermal proliferation of disorganized and diffuse collagen fibers (green). a.) 4x. b.) 10x.
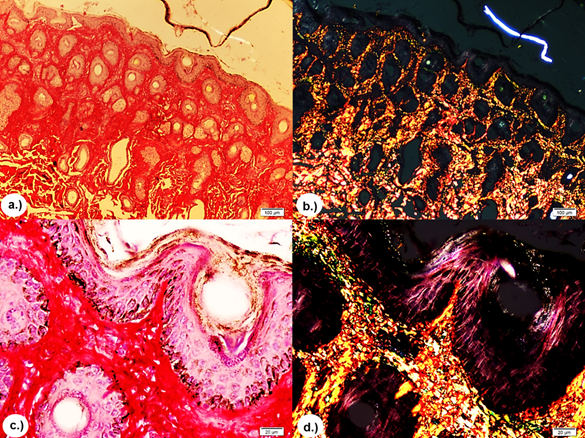
Figure 5 P-R/P stain without polarization and with polarization, respectively. Showing areas of reddish and yellowish-green birefringence indicating moderate presence of mature collagen type I (bright red in polarization) and type III (bright green-yellow in polarization). a.) and b.) 4X. c.) and d.) 10X.
All animals recovered 30 to 40 days after initiation of treatment, so there is no necropsy or hepatic histopathology data, and it was not possible to achieve special "Tru-Cut" needles to perform liver biopsies.
DISCUSSION
There are no reports in consulted literature on secondary phototoxic dermatitis in cattle in the department of Córdoba and Colombia that provide data on the time of year, type of Brachiaria in these cases, age at onset, duration of exposure and characterization of lesions, so this report is an important contribution to the knowledge of the disease in the region and the country.
DFS caused by Brachiaria spp. is likely to be sub-reported either for mortality or treatments by owners or operators. However, it is a major cause of damages that have a great economic impact on production systems around the world, especially in Brazil where the majority reports and research has been done 22. Therefore, economic losses can be direct in the case of death of an animal with DFS or in decreased production rates (abortions, infertility, loss of weight or milk, meat, etc.) or indirectly due additional pasture management strategies, fence utilization for rotational grazing, reduction of pasture quality by delaying use, replacement of dead animals and expensive treatments 23.
The most common clinical signs were photophobia, edema around the eyes and chin, cracking, peeling and scabbing of skin around ears, lower jaw, perineal, costal and inguinal regions and progressive emaciation. All these are similar to those described by Mustafa et al (1, Sant'Ana et al 2, Riet-Correa et al 7,8, Oliveira et al 9, Souza et al 10, Albernaz et al 11, Quinn et al 12, and Faccin et al 14. Lesions were located at sites of increased exposure to sunlight according to what was reported by Sant'Ana et al 2 and Oliveira et al 9; however, Porto et al 5 expressed that sun exposure does not determine the presentation of clinical signs of intoxication but exacerbates clinical symptoms in affected animals.
Riet-Correa et al 7, show that progressive emaciation is due to malabsorption associated with the presence of foamy macrophages in the intestinal mucosa, which leads to weight loss. Jaundice observed in the clinical examination is a sign of hepatoxic injury caused by ingestion of Braquiaria and was confirmed by increased AST and GGT serum activity due to extravasation of bile pigments from bile ducts obstructed by crystals formed by conjugating saponin with calcium 7,18. Keratitis and blindness were not observed in the calves studied as clinical signs of B. decumbens poisoning, as reported by Albernaz et al 11 in sheep poisoned by B. brizantha in the state of Pará (Brazil).
The high catalytic activity of liver enzymes is consistent with what was reported by Mustafa et al 1, Sant'Ana et al 2, Porto et al (5, Riet-Correa et al 7, Oliveira et al ( 9), Souza et al 10, Albernaz et al 11 and Faccin et al 14, who found that animals with DFS manifest skin lesions and present a sharp rise in AST and GGT liver enzymes, indicating that this finding is of great diagnostic value for liver injuries in animals poisoned by Brachiaria spp. grasses. Similarly, they explain that GGT is an enzyme that exhibits increased activity in liver diseases associated with cholestasis since it is linked to cells membranes and is mainly located in canaliculi, bile ducts, and to a lesser extent in hepatocytes and is released when there these structures are damaged. Similarly, Gracindo et al 6 and Faccin et al 14 reported in their study that the mean serum catalytic activity of GGT in sheep that grazed on B. decumbens was higher than sheep that grazed in paddocks with other grass.
Clinical manifestations and mortality in cases of poisoning by Brachiaria spp. depend on factors related to the plant and factors associated with susceptibility or resistance of the animal 8. According to what has been observed, sheep grazing on Brachiaria spp. can have high GGT elevations without presenting clinical manifestations of intoxication 24, suggesting that are resistant to poisoning with subclinical or absent expression 7. Therefore, the level of GGT activity is considered a good indicator of Brachiaria spp. poisoning. As was demonstrated, greater GGT activity in animals never exposed than those exposed for long periods to Brachiaria spp. pasture suggests GGT can be a good indicator of resistance, and similarly, high concentrations of GGT without clinical signs of toxicity in animals exposed for long periods to pasture 7,8. Therefore, a way to increase resistance in the flock is eliminating animals that exhibit clinical signs of the disease or by monitoring GGT concentrations in exposed individuals 5,14.
Histopathologic findings of this study with the H&E stain correspond to those reported in literature to diagnose DFS 4,5,14, especially in foci of necrosis of the epidermal layer, neutrophilic infiltration and hyperkeratosis. Similarly, in the T&G strain, the presence of diffuse connective tissue with small areas of poor dermal fibroblast proliferation and the presence of disorganized collagen fibers was evident. Also, in the P-R/P stain small areas of red and yellow-green birefringence were observed, indicating a moderate presence of mature collagen type I and type III, which could indicate moderate regenerative response against aggression phototoxic process. These techniques have been used as a response indicator of skin repair in aggressive skin states 25. However, it is necessary to clarify that there are no reports of the use of histochemical techniques such as T&G and P-R/P to diagnose and prognosis of DFS in cattle.
The calves in the study were between 12 and 18 months of age, coinciding with what by Souza et al 10 reported, who grouped photosensitizing animals in that age range, and in a similar manner Sant'Ana et al 2 reported greater presentation in calves between 4 and 14 months and a lesser tendency in adult cattle.
The animals recovered 30 and 40 days after initiating the treatment strategy, which agrees with Gracindo et al 6 who reported that recovery time of the affected cattle was between 15 and 45 days after being removed from the areas with Brachiaria spp.; however, it states that it is likely that the animals have cutaneous scars until slaughter.
From a toxicological point of view, Brachiaria is often classified as a toxic photosensitizing plant 26, since all Brachiaria have shown some degree of toxicity. In his study Sant'Ana et al 2 found greater poisoning outbreaks with Brachiaria in grazing on B. decumbens, followed by B. brizantha and B. humidicola in minor proportions, coinciding with that reported by Riet-Correa et al 7, who showed that B. decumbens has a higher concentration of saponins than B. brizantha and B. humidicola.
The group of affected calves came from areas free of Brachiaria and were put to graze in a pasture that had been planted 2 months prior with Brachiaria decumbens, which may be a risk factor for the outbreak, consistent with that reported by Gracindo et to 6 and Lima et al 27, who found higher mean values of protodioscin saponin at 60 days in B. decumbens, indicating that young plants contain four times more saponin protodioscin than old and mature plants. Some outbreaks of DFS occur when animals are placed in paddocks that have been unused for some time, after the rainy season or during the period of regrowth as happened in the months in which the study was conducted, which coincides with the first rains on the northern coast of Colombia. However, Brum et al 28) show that the highest concentrations of protodioscin saponins are found in the maturation phase of Brachiaria, coinciding with the fall of seeds.
Because of the importance of Brachiaria spp. in many regions in tropical countries for its nutritious excellence as forage and its ability to grow in more rugged terrain, it is very important to establish preventive practices and measures to control toxicity using less toxic Brachiarias varieties with decreased protodioscin concentration than B. decumbens1,7,27. Similarly, the use of resistant animals is recommended by selecting those that did not have signs or that maintained liver enzyme levels in the normal range for the species.
In conclusion, the clinical features of skin lesions were consistent with the results of serological tests and histopathological findings in twelve cattle in the department of Córdoba with the presence of DFS. It is an important contribution to confirm accurate diagnosis of pathological conditions and to warn about the presentation of the disease and the need for further studies on this pathological condition in areas where only Brachiaria spp. is available, and its effects on cattle in the region.














