INTRODUCTION
In South America, tilapia production has grown in the last decades 1. The cichlids present notably characteristics for production, such as rapid growth, tolerance to a wide range of environmental conditions, available for eating a variety of foods, white meat and relatively low production costs 2,3.
The intensification of tilapia production and high stocking densities are favourable conditions to spread diseases 4; and that induce inflammatory process, dramatically compromising the immune response 5,6 ending with the death of the animal. Currently several epizooties that affect them, usually caused by bacterial agents are known 2. The Streptococcal infections have become a major problem in tilapia production, generating high morbidity and/or mortality, and severe economic losses in different fish farms in the world 3,7.
Streptococcus agalactiae and Streptococcus iniae are the main bacterial species that affect production of tilapia in the world (8). However, some authors mention that the S. iniae is more distributed, affecting a larger number of fish species than S. agalactiae9. S. iniae causes high rates of mortality, which varies between 30-50% 9. Also, this agent causes changes in fillets, which are inappropriate for trade 2. In addition, it has been reported that S. iniae is a zoonotic pathogen 10.(3/16)
In Peru, the tilapia production is a promising activity. However, there is a lack of identification and knowledge of bacterial diseases that affect this culture and could impair its production (11). Previous studies reported the existence of clinical signs and lesions consistent with Streptococcus spp. 12.
The aim of this study was to determine if S. iniae is present in an intensive fish farm of Oreochromis niloticus located in Sullana-Piura, Peru.
MATERIALS AND METHODS
Experimental design. 150 tilapias were collected 75 of the phases nursery (33.5±3.6 g) and 75 grow-out (670.5±22.6 g) from ponds with clinical signs such as shallow and erratic swimming, exophthalmia and haemorrhagic skin lesions. The animals were euthanized with a lethal dose of sodium benzocaine 1:500 (v/v) previously diluted in alcohol 98° (0.1 g/mL) 13.
Location. The fish were sampled from an intensive fish farm located in the Lancones district, Sullana province, Piura department, to Peru north-western (4 ° 38’27 “S 80 ° 32’55” W). The water surface area is 19.26 hectares (Ha) and is supplied by the Poechos reserve. Flow areas and the fish farm are: reproductive reception, reproduction, breeding 1, breeding 2, growth, nursery, grow-out and harvest.
The study was performed between the months of February and June in 2014, with at room temperature between 24°C - 35°C. Also, the averages of the physic-chemical parameters of the water recorded for the fish farm (using a multiprobe system, model YSI-556) were:
Nursery: T°: 25.3±1.8 °C, Dissolved oxygen (DO): 5.3±0.6 mg/L, CO2: 4.1±0.9 mg/L, pH: 7.3±0.6, nitrite: 0.008±0.003 mg/L, ammonium: 0.14± 0.02 mg/L, alkalinity: 92.07±2.6 mg/L, hardness: 92.5 ±3.2 mg/L.
Grow-out: T°: 25.1±2.2 °C, Dissolved oxygen (DO): 3.99 ±0.9 mg/L, CO2: 2.93 ±0.4 mg/L, pH: 5.7±0.4, nitrite: 0 mg/L, ammonium: 0.34±0.12 mg/L, alkalinity: 98.84±3.1 mg/L, hardness: 93.2±2.8 mg/L.
Sample size. To determine the sample size was used the limit prevalence formula for detection of a disease. The use of this formula means that there is a probability of the 95% to find at least one positive, if the prevalence is equal or greater than the limit prevalence (2.7%). Consequently, the absence of positive indicates that the disease to be present, its prevalence would be less than the limit prevalence.
Ethical aspects. The research was approved by the Committee of Ethics and Animal Welfare - CEAW (CEBA2005-009), School of Veterinary Medicine of the National University of San Marcos, according to compliance with international principles and laws as outlined by the Institutional Animal Care and Use Committee (IACUC).
Microbiology. Aseptic court of the indicated organs (spleen, brain, liver, and head kidney) was performed and the sterile swab from Stuart transport medium (Deltalab, SLU, Spain) was introduced. The swab samples were seeded in blood agar plates (supplemented with 5% sheep blood) and incubated at 30°C for 24-48 hours.
Colonies were selected that showed characteristics of Gram positive cocci, small colonies white, translucent or pigmented, slightly convex, up to 2 µm in diameter, with negative testing for catalase, oxidase and motility some haemolysis, glucose (Neogen Acumedia, USA), lactose (Sigma, USA), maltose (Sigma, USA), mannitol (Merck, Germany), indole and H2S production. In addition, were considered the following tests to differentiate the species S. iniae: acetoin production (Voges Proskauer) (Remel, USA), aminopeptidase leucine (LAP) (Remel, USA), arylamidase pyrrolidonyl (PYR) (Remel, USA), starch hydrolysis (Merck, Germany) and esculin hydrolysis (Neogen, Acumedia, USA).The protocol for the tests mentioned followed the manufacturer’s recommendations.
Histopathology. Tissues cutting about 1.0 cm diameter were fixed in 10% buffered formalin solution. Samples of spleen, gills, brain, cecum, heart, stomach, liver, intestine, skeletal muscle, skin and head kidney were processed according to the standard protocol for histological studies of fixed tissues. Cross-sections with thickness of 5 µm were fixed and stained with hematoxylin-eosin.
Molecular Biology.
DNA extraction. The DNA extraction was performed from pools of organs of each fish of the presumptively positive of S. iniae by biochemistry, with the commercial kit PureLink Genomic DNA Kit (Invitrogen, USA), following the manufacturer’s instructions for Gram positive bacteria. For DNA purification step centrifugation at 13,000 x g was performed for 1 minute followed by two washes with the elution buffer, using 40 µL each wash. DNA concentration and absorbance ratio (260/280 nm) were measured using a spectrophotometer (Nanodrop, Thermo Scientific, Waltham, MA, USA). Finally, the genetic material stored at -20°C until use.
Controls. The strains used as positive controls were S. iniae ATCC 29178 donated by the Laboratory of Genomic Medicine of the Surcolombiana University and the S. iniae ATCC 29177 (MicroBioLogics, USA). Both extracted with the commercial kit PureLink Genomic DNA Kit (Invitrogen, USA). Furthermore, water DEPC (Invitrogen, USA) was used as negative control.
Qualitative real-time PCR. The primers and probe used for the detection of S. iniae were forward 5’GTTTTCTTGAAGCTATTGCAGGTT T3’, reverse 5’CGCGCAAGGGTTTCATG3’ and prove 5’VIC- CTGCGGTTGCCATACCAGCAAGTATTCTA-TAMRA3’. These primers and probe were synthesized from the sequence No. Y07622.1 lactate oxidase protein (lctO) using the Primer Express 3.0 Applied Biosystems Software (Life Technologies, USA). PCR was performed in duplicate, in short: 12.5 µL of Taqman Universal PCR Master Mix (2x) (Invitrogen, USA), 0.5 µL of probe (10 µM) (Bioneer Corporation, Corea), 1.25 µL of primer F (10 µM), 1 25 µL of primer R (10 µM), 1 µL of DNA (20 ng/µL), 8.5 µL of DEPC water (Invitrogen) to a total volume of 25 µL.
Thermal conditions used were: denaturation at 95°C for 10 minutes followed by 40 cycles of 15 seconds at 95°C, 2 minutes annealing at 50°C and 1 minute of extension at 60°C using the equipment Applied Biosystems 7500 (Life Technologies, USA).
Data analysis. The calculation of the prevalence of positive to Streptococcus spp. tilapia was conducted. For microbiological characterization and tilapia S. iniae alleged positive by biochemical, by @Risk (Palisade Corp) uncertainty program implemented in Excel® spreadsheet (Microsoft Corp).
For the calculation of the prevalence of S. iniae in sick animals or with clinical signs was developed a stochastic simulation using beta distribution program @Risk Uncertainty (Palisade Corp) implemented in a spreadsheet Excel® (Microsoft Corp.). Briefly, the probability of infection in apparently sick fishes and their intervals were calculated using a Beta distribution with parameters α and β where:
α=positivos+1 and β=negativos+1
Confidence intervals were calculated running the simulation 30.000 interactions.
RESULTS
Necropsy. The post-mortem findings evidenced pathological changes such us exophthalmia, ocular opacity, congestion of meninges, ascites haemorrhagic lesions in different zones of the body, bleeding anal pore, splenomegaly, hepatomegaly and necrotic zones on the skeletal muscle (Figure 1a-e).
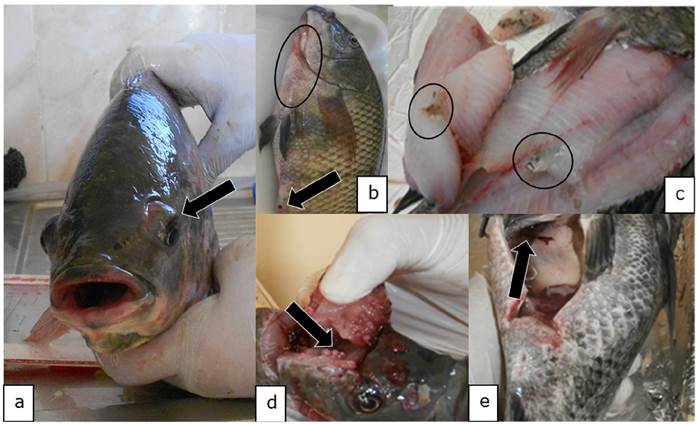
Figure 1 Macroscopic findings compatible with Streptococcus spp. in in Oreochromis niloticus. a) Exophthalmia (arrow). b) Haemorrhagic lesions on skin (circle) and bleeding anal pore (arrow). c) Necrotic lesions on skeletal muscle (circle). d) Congestion of the brain (arrow). e) Ascites (arrow).
Microbiology. 102 positive isolates were identified as Streptococcus spp. The distribution of these isolates by organ were: liver (18) 17.65%, head kidney (22) 21. 57%, spleen (27) 26.47% and brain (35) 34.31%. These isolates corresponded to a total of 54 positive tilapia to Streptococcus spp.
From 102 isolates were selected (n=19, 18.63%) only those with ß-haemolytic, characteristic of S. iniae. These isolates belong to a total of 16 tilapias. The Table 1 shows the biochemical profile of these isolates.
Table 1 Biochemical profile and microbiological characteristics from 19 isolates of Streptococcus spp. of Oreochromis niloticus from fish farm in Sullana-Piura, Peru.
| Characteristics | Isolated | % |
|---|---|---|
| ß-Haemolysis | 19 | 100% |
| CAMP | 19 | 100% |
| Starch hydrolysis | 13 | 68.42% |
| Voges-Proskauer | 10 | 52.63% |
| LAP | 14 | 73.68% |
| Mannitol | 11 | 57.89% |
| Maltose | 16 | 84.21% |
| Esculin | 11 | 57.89% |
| PYR | 3 | 0.16% |
| Glucose | 18 | 94.73% |
| Lactose | 14 | 73.68% |
| Indole | 9 | 47.37% |
| H2S | 17 | 89.47% |
The average prevalence of positive tilapia to Streptococcus spp., by microbiological characterization was 26% (21% - 33%). Whereas, the average prevalence of positive presumed tilapias to S. iniae by biochemical profile was 10.12% (6% - 15.10%).
Histopathology. To the histopathological reading of the 16 tilapia positive presumed to S. iniae was observed:
Spleen: In 12.5% (2/16) of the cuts, the perisplenic area was observed thickened with the presence of inflammatory exudate composed of neutrophils and macrophages on condensed fibrin, and in some cases, the presence of fibroblasts; compatible with a fibrin-suppurative acute or chronic type perisplenitis (Figure 2-a). In 50% (8/16) bacterial nests and areas of necrosis were noted. In addition, other cuts congestion showed 68.75% (11/16) in the depletion 18.75% and increased melanomacrophages centres in 43.75% (7/16).
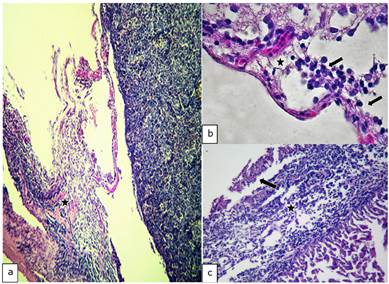
Figure 2 Histopathological findings in Oreochromis niloticus. a) Photomicrograph of the spleen showing a fibrinosupurative splenitis (star). b) Photomicrograph of the brain showing congestion (star) and mononuclear infiltrate (arrow). c) Photomicrograph of the heart showing of the thickened epicardium (star) and mononuclear inflammatory exudate on fibrin deposits (arrow). Hematoxilin-Eosine, Scale bar = 20 µm.
Brain: In 37.5% (6/16) of the cuts acute diffuse suppurative meningitis was observed. While, in 18.75% (3/16) was observed an infiltration of the brain with an exudate composed of neutrophils and macrophages, consistent with meningoencephalitis. In addition, congestion meninges in 18.75% (3/16) and 6.25% (1/16) for the presence bacterial nests were evidenced (Figure 2-b).
Heart: Severe acute fibrinous suppurative epicarditis was observed and in other cases, chronic type, shown in 56.25% (9/16). In some compact infarction disintegration was observed cardiac muscle fibers, including the presence of inflammatory exudates comprising neutrophils, macrophages, eosinophilic granule cells (ECG) and red blood cells, representing 56.25% (9/16) (Figure 2-c).
Stomach: Suppurative gastritis was evidenced in 18.75% (3/16).
Liver: The thickened perihepatic area was observed with the presence of an inflammatory exudate composed of neutrophils (some or degenerate), macrophages and eosinophilic granule cells seated on fibrin condensed and in other cases, the presence of fibroblasts was demonstrated. These lesions were compatible with a severe acute suppurative fibrin-perihepatitis and in other cases, chronic type, in 37.5% (6/16). A cut to 6.35% (1/16) showed the presence of bacterial nests.
In 12.5% (2/16) of the cuts granulomas, composed by aggregated macrophages which were circumscribed by fibroblasts. Also, the hepatopancreas showed an inflammatory exudate (Figure 3-4) it was evidenced. Moreover, 75% (12/16) of sample fish showed necrosis. While in 56.25% (9/16) steatosis and hydropic degeneration of hepatocytes was evidenced.
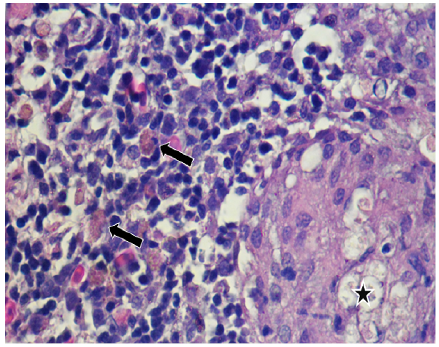
Figure 3 Histopathological findings in Oreochromis niloticus. Photomicrograph of the hepatopancreas showing a granulome with aggregated macrophages (star) and free melanomacrophages (arrows). Hematoxilin-Eosine, Scale bar = 20 µm.
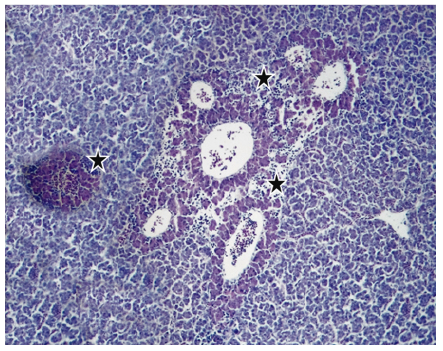
Figure 4 Histopathological findings in Oreochromis niloticus. Photomicrograph of the hepatopancreas showing inflammatory exudate around of the hepatopancreas (star). Hematoxilin-Eosine, Scale bar = 20 µm.
Intestine: Infiltration of the intestinal epithelium, presence of neutrophils, macrophages and melanomacrophages were observed representing 6.25% (1/16) of cases. Serosa macrophage infiltration was also observed at 6.25% (1/16) of cases. Cutting a 6.25% (1/16) plus epithelial hyperplasia and necrosis was observed; compatible with mild to moderate acute diffuse suppurative enteritis.
Skeletal Muscle: Coagulative necrosis was observed representing 56.25% (9/16) of the cases (Figure 5), presence of some inflammatory cells (macrophages, neutrophils and eosinophilic granule cells in 6.25% (1/16) of cases.
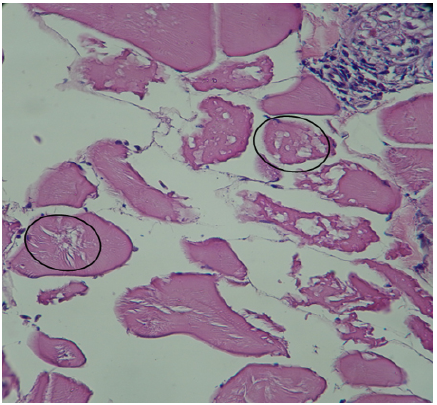
Figure 5 Histopathological findings in Oreochromis niloticus. Photomicrograph of the skeletal muscle showing coagulative necrosis (circle). Hematoxilin-Eosine, Scale bar = 20 µm.
Eyes: The layers of the retina were observed disintegrated, demonstrating a process of oedema in 31.25% (5/16). The corneal epithelium, choroid plexus and adipose tissue were observed severely infiltrated with presence of exudate composed of neutrophils and macrophages (panoftlamitis and panniculitis), compatible with moderate to severe acute suppurative panophthalmitis, representing 81.25% (13/16) of cases (Figure 6).
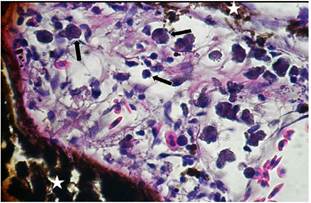
Figure 6 Histopathological findings in Oreochromis niloticus. Photomicrograph of the eye showing macrophages, neutrophils (arrows) and cells of the pigmentary epithelium (star). Hematoxilin-Eosine, Scale bar = 20 µm.
Thirteen cuts with 81.25% (13/16) showed scattered cells of the retinal pigment epithelium, showing phagocytic activity.
Skin: The skin hydropic degeneration and necrosis with loss of epithelium was observed in a cut representing 6.25% (1/16). Furthermore, the dermis and infiltration of debris exudate comprising inflammatory cells (neutrophils and macrophages), consistent with acute suppurative dermatitis was observed, and representing 12.5%(2/16) cases.
Molecular Biology. From 16 tilapia characterized by biochemistry and histopathology positive presumed to S. iniae, any positive tilapia was found to real time PCR. However, it was estimated by uncertainty @Risk program (Palisade Corp), the mean prevalence of 0.66% (0.02% - 2.41%) for S. iniae in diseased tilapias or with clinical signs.
DISCUSSION
Peru has excellent prospects for aquaculture development and within this framework; the farming of tilapia is a promising activity. Also, in recent years it has increased consumption and acceptance of tilapia, in national and international markets 11. It is important to ensure optimal health status and provide a safe product to the consumer. To date, in Peru there are only reports of parasites present in this species of reared 12. So, this study represents some of the first reports on the identification of bacterial agents in tilapia.
The streptococci is one of the most important bacterial diseases affecting fish farming, and within species, S. iniae is considered the greatest impact on tilapia farming 9. Due to the morbidity, mortality and treatment costs it generates, and its impact on public health 13,14.
The main macroscopic findings in this study such as exophthalmos, corneal opacity, congestion in the brain, ascites, the bleeding anal pore, haemorrhagic and necrotic lesions on the skin and muscle tissue, liver, spleen, fibrin deposits in liver and heart were suggestive of Streptococcus spp. infection 8.
The 34.31% of isolates of Streptococcus spp., from the brain found in this study supports the predilection of this bacterium by this organ as suggested by Salvador et al 15, considering it an important organ for routine isolation. Also, it was determined that the spleen, kidney and liver are important organs for isolation of Streptococcus spp., which was 26.47%, 21.57% and 17.65% respectively.; these organs are also considered by Hernandez et al 16 as targets for their isolation. Even if in this study was not found a biochemical profile 100% typical to S. iniae, according to Anshary et al 17, we can infer that is due to the different variations between isolates as reported by Facklam et al 18.
Histopathological lesions observed in this study are consistent with infection by Streptococcus spp. The most affected tissues were: heart, liver, eye, spleen and brain; these organs are reporting that they would be the target organ of the bacteria, according to that found by Salvador et al 15.
The presence of epicarditis fibrinous, fibrinous perisplenitis, meningitis, hepatopancreatic granulomas, bacterial nests, necrotic foci, infiltration and presence hepatopancreas melanomacrophages centres is also reported by Chen et al 19 in positive tilapia to S. iniae, which until histopathology, could lead us to assume that the specie could be present in this fish farm. Also the ophthalmitis or panophthalmitis indicated the predilection of Streptococcus spp. for this organ, as was observed by Bromage and Owens 20. Furthermore, the exudate observed in all organs is mainly mononuclear, predominantly macrophages and neutrophils.
The 16S rRNA gene is usually used for the detection of S. iniae. However, Gibello et al 21 found that the gene encoding the protein lactate oxidase (lctO) is very unusual between species of Streptococcus spp.; for it, this gene is able to detect S. iniae with greater specificity than the 16S rRNA gene.
This study is the first to determine the presence of the genus Streptococcus spp., supported by microbiological isolation and pathological lesions in the Sullana province, Piura department, Peru. It can be concluded that the species S. iniae was not present in the specimens of O. niloticus analysed in this fish farm to the limit prevalence of 2.7%. However, is necessary to confirm the species of Streptococcus found in this important farming centre for our country; because the clinic signs and lesions are not pathognomonic for Streptococcus infection, for this becomes necessary the use of molecular identification.














