INTRODUCTION
Liseta Leporinus muyscorum1 is a fusiform characiform fish that has three or four dark spots on its body when it reaches adulthood 2. The maximum length collected for the species downstream of Urra´s dam is 44.0 cm TL in 2002 year, while its maximum length theoretical in the Sinu river basin 45.7 cm TL, with medium longevity and growth rate 3.
Leporinus muyscorum is endemic from Colombia, from Atrato, Truando, Magdalena, San Jorge and Sinu river basins 4, inhabit basically in the main channel, moving through the caños in search of food or dispersal areas or gonadal maturation. In the Atrato river is an omnivorous species with herbivorous tendency, preferring a diet of leaves, fruits, grains and seeds 5.
It is one of the rheophilous fishes from the Sinu river basin that has two annual migrations that makes it susceptible to fishery exploitation, both when move upstream as well as when to return to feeding areas, so it is one of the species commercially important in the Sinu river basin fishery. His capture in the basin increased considerably due to the decrease in fishery production of traditional species such as Bocachico Prochilodus magdalenae and Blanquillo Sorubim cuspicaudus has allowed an increase in catches of lower commercial value 3.
The aim of this work was to study the reproductive biology of Liseta in the Sinu river as a contribution to the state of knowledge of the species, its conservation in the natural environment, the management of their fishery and the establishment of a database for his production in controlled cultures.
MATERIALS AND METHODS
Location and description of the study area. The study took place in the Lower Sinu river between March 2006 and February 2007. The Sinu River rises in the Nudo de Paramillo, Paramillo Natural National Park, in the Ituango´s municipality (Antioquia department) at 3700 m.s.n.m, and runs to its mouth in the Delta Tinajones in the Caribbean Sea, Cordoba department. The total length of the channel is 437.97 km, passes through the territories of Ituango, Tierralta, Valencia, Montería, Cereté, San Pelayo, Cotorra, Lorica and San Bernardo del Viento, with annual average temperatura of 27 ºC and multianual average rainfall of 1300 mm 6.
Obtaining the Samples. For taxonomic identification, Garavello 2 was followed. 334 individuals were collected and total length (TL) were recorded to the nearest millimeter with a graduate ichthyometer (IK2, Aquatic Biotechnology, Spain) and total weight (TW) to the nearest gram with an electrical scale of 5000 ± 1 g (CS 5000, Ohaus Corporation, USA). Sampling were carried out using cast nets and hook and line and the collected specimens were cooled and transported to the Fisheries Biology Research Laboratory-FBRL of Cordoba University at Lorica.
Biological material. The fishes were eviscerated and the gonads were separated from other organs and weighed on a 1500±0.01 g electrical scale (Adventurer, Ohaus Corporation, USA) and preserved in Gilson solution. The Vazzoler´ scale 7 was applied.
Analysis of reproductive biology. The sex ratio was calculated using the equation: % males=100 * (Nm/Nt) 8 where Nm is the number of males and Nt the total number of individuals, and sexual proportion to size with the Holden & Raitt 9 technique using class intervals of 2.0 cm.
The gonadosomatic index (IGS1) and the corrected gonadosomatic index (IGS2) were estimated: IGS1=100*GW/TW 10, where GW is the weight of the gonads (ovary or testes) and TW is the total weight of the fish; IGS2=100*GW/EW 10 where WE is the eviscerated fish weight. The gonad index (IG) was also obtained: IG=104*GW/TL 7, where TL is the total size of the fish and b is the growth rate of the length-weight regression.
The size at sexual maturity was estimated using the methodology proposed by Sparre & Venema 11. The diameter of 1236 oocytes from different samples from every month of the year, selected at random, was measured using an ocular micrometer to establish the frequency distribution of oocyte diameter and to select mature ones.
The spawning season was estimated taking into account the macroscopic analysis of the ovaries and testis, the collection of mature and spawned females at four and seven months of the study, respectively, the collection of mature and sperm producing males at five and seven months of the study, respectively, the sexual maturity index maturity, the diameter of mature oocytes and the statistically significant differences found between the four states of sexual maturity assigned for each sex.
A subsample of each sexually mature type III ovary (0.15-0.25 g) was taken using gravimetric analysis 12 to estimate total fecundity (F) of the species under study: F=nG/g, where n is the number of mature oocytes in the sample, g is the weight of oocytes and g is the weight of the sample. Additionally, total length-fecundity, total weight-fecundity and ovary weight-fecundity equations were estimated.
The variables studied are presented as mean ± standard deviation. The statistical chi-square test was used to confirm whether the estimated sex ratio was in line with expectations and the analysis of variance to assess changes in the estimated rates for females and males at each sexual maturity stage during the reproductive cycle analyzed. The Tukey-Kramer multiple comparison test was used when statistically significant differences were found.
RESULTS
344 individuals were analyzed with sizes and weight between 20.5 - 41.0 (30.0±3.7) cm LT and 97.6-728.0 (320.9±117.9) g, of which 249 were females and 95 males (Table 1). The minimum size was recorded in April and the maximum in May, and the size frequency distribution showed a normal curve with average catch length of 30.2 cm TL (Figure 1).
Table 1 Sexual proportion of Liseta (Leporinus muyscorum) according to size in the Sinú River.
| Size (cm LT) | Females | Females (%) | Males | Males (%) | H : M | X 2 (Obs.) | X 2 (Tab.) |
|---|---|---|---|---|---|---|---|
| 17.5 - 22.5 | 2 | 66.7 | 1 | 33.3 | 2.0:1 | 0.333 | 3.841 |
| 22.5 - 27.5 | 54 | 79.4 | 14 | 20.6 | 3.9:1 | 23.529 | 3.841 |
| 27.5 - 32.5 | 139 | 70.9 | 57 | 29.1 | 2.4:1 | 34.306 | 3.841 |
| 32.5 - 37.5 | 42 | 65.6 | 22 | 34.4 | 1.9:1 | 6.250 | 3.841 |
| 37.5 - 42.5 | 12 | 92.3 | 1 | 7.7 | 2.9:1 | 9.308 | 3.841 |
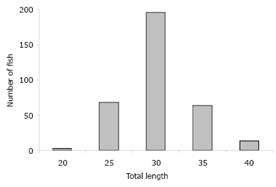
Figure 1 Distribution of fish size frequencies for Liseta in the Sinú River. March 2006-February 2007.
The female: male sexual proportion observed was 2.6:1 different from the expected 1:1 (X2: 68.942; p:0.05; 1 gl). Analyzed monthly, significant statistical differences were found almost every month of the year except May 1.5:1 (X2: 0.926; p:0.05; 1 gl), June 0.9:1 (X2:0.048; p:0.05; 1 gl) and October 1.5:1 (X2: 1.200; p:0.05; 1 gl). The female: male sexual proportion in size (Table 1) was different from expected in all intervals except the smallest (17.5-22.5) cm TL (X2:0.333; p:0.05; 1 gl).
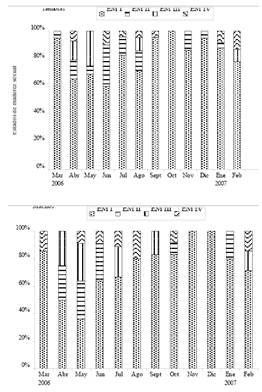
Most of the sample consisted of immature individuals, both females (n =206, 82.7%) and males (n =70, 73.7%). Mature females (maturity stage III) were collected during four months of the study (April, May, August and February), with the greatest numbers in April and May with 4 individuals in each month, and spawned females (maturity stage IV) in 7 months of the study (April, June, July, August, September, January and February). The highest values of the three indices were found in May (Figure 2). Mature males were collected during five months of the year (April, May, July, September and February), especially in May with 3 individuals, and sperm off individuals during seven months (March, May, June, July, August, October, February), mainly in April where highest values in the three indices was found (Figure 2) were found.
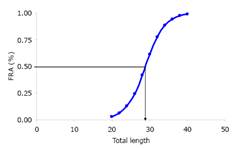
Table 2 presents the gonadosomatic index 1 (GSI1), gonadosomatic index 2 (GSI2), and the gonadal index (GI) for females and males, as well as the annual average, respectively. The length at first maturity average size at maturity was estimated at 28.9, 28.1 and 28.8 cm TL for females, males and combined sexes (Figure 3), respectively.
Table 2 Average values of sexual maturity index of Liseta females and males in the Sinú River.
| State of maturity | n | IGS1 | IGS2 | IG |
|---|---|---|---|---|
| I | 204 | 0.426 ± 0.26 a | 0.519 ± 0.31 a | 0.00008 ± 0.00005 a |
| II | 20 | 0.581 ± 0.22 a,c | 0.691 ± 0.26 a,c | 0.00011 ± 0.00004 a,c |
| III | 12 | 2.444 ± 1.13 b | 3.189 ± 1.73 b | 0.00050 ± 0.00025 b |
| IV | 11 | 0.893 ± 0.40 c | 1.091 ± 0.49 c | 0.00016 ± 0.00008 c |
| I | 70 | 0.074 ± 0.06 a | 0.089 ± 0.07 a | 0.00001 ± 0.00001 a |
| II | 8 | 0.225 ± 0.04 b | 0.266 ± 0.04 b | 0.00003 ± 0.00001 b |
| III | 8 | 0.329 ± 0.08 c | 0.402 ± 0.10 c | 0.00005 ± 0.00001 c |
| IV | 8 | 0.184 ± 0.16 b | 0.233 ± 0.21 b | 0.00003 ± 0.00003 b |
| n =number of individuals per maturity stage in the reproductive cycle. IGS1=Gonadosomatic index. IGS2= Corrected gonadosomatic index. IG=gonad index. Values with equal letters in the same column are statistically similar. |
Two groups of oocytes were found in the ovaries, one that corresponded to the batch of mature oocytes and that would be spawned, and another batch that was the reserve stock, indicating a synchronous oocyte development in two groups with a spawning season among February and September. The mature oocytes are large with a diameter that ranges between 901 and 1239 μ, with an average of 977 (±77) μ.
The weight of ovaries ranged between 4.67 and 35.16 (10.78±8.05) g, which corresponded to individuals with sizes and total weights among 28.5 and 41.0 (33.3±4.9) cm TL and 284.0 and 655.5 (427.7±118.8) g. It was found that while the size and total weight are homogeneous (CV<30%), the weight of the ovaries is heterogeneous (CV=87.6%), which partially explains the dispersion in fecundity estimate (CV=77.9%).
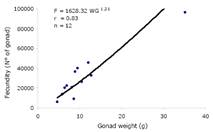
Average fecundity estimated was 30793±23976 oocytes, with a maximum of 96350 eggs for an individual with 39.2 cm TL, 655.5 g, and gonad weight of 35.16 g. The average relative fecundity was 935 ±651 oocytes/cm TL, 72±44 oocytes/g, and 2798±997 oocytes/g of gonad. The best equation was: F=1628.32 WG 1.21, r=0.83, n=12 (Figure 4), where the correlation coefficient is high and statistically significant at 95% confidence.
DISCUSSION
The female: male ratio of this study (2.6:1) was different from what was found in the Paraná River for Leporinus obtusidens, where a predominance of females (61.0%) to males (39.0%) was observed with a sex ratio of 1.6:1 13, and the study in the Mesay River (Colombian Amazon) of L. brunneus, with a predominance of females (29 fish, 88.0%) over males (12 fish, 12.0%) and a sex ratio of 7.3:1; L. fasciatus, predominantly female (8, 80.0%) over male (2, 10.0%) and a sex ratio of 4:1; and L. klausewitzi predominantly male (11, 64.7%) over female (6, 35.3%) and a sex ratio of 1.8:1 14.
Segura et al 15 claim that the geographic areas where a particular fish species lives, the compared distance between different areas, population growth rhythm or rate, fishing gear used, number of fish collected, and other factors explain the differences in the size of fish collected, their average catch size, and the sex ratio.
As expected, rates of sexual maturity reached their highest values in state III (2.44%), as happens in most bony fish 7, finding statistically significant differences (p <0.05) between this state and the other three, both in gonadosomatic index 1 and gonadosomatic index 2 and the gonadal index for females and males (Table 2). Statistically significant differences were also found for females of L. copelandii (IGS1=5.55%) in the Paraiba do Sul River (Brazil) 16 and for L. taeniatus females (IGS1=11.41) and males (IGS1=3.29%) in the Juramento reservoir (Brazil) 17.
Other studies done in Brazil show that the maximum gonadosomatic index 1 in mature females in some species of the Anostomidae family reached 14.42% (Leporellus vittatus), 15.25% (Leporinus amblyrhynchus), 11.06% (L. elongatus), 22.83% (L. friderici), 21.77% (L. lacustris), 8.72% (L. obtusidens), 21.48% (L. striatus), 14.25% (Schizodon borellii) and 15.38% (S. nasutus) 7. It was observed that all are above the maximum value found in this study (5.36%), which is associated with the state of sexual maturity and fecundity of each particular species.
The average size at sexual maturity estimated in this study, 28.9, 28.1 and 28.8 cm LT for females and males and both sexes combined, respectively, are lower than those reported for the species in the Sinú River (24.5 cm LS, 30.5 cm LT), and are higher than average catch sizes (TMC=25.8 cm LT, n=4300) reported in the Sinú River basin 3, which indicates that the species is being fished long before it reproduces. However, these average sizes at sexual maturity are lower than the average catch size estimated in this study (TMC=30.2 cm LT).
The results are an important input that can be used by fisheries authority in Colombia (AUNAP) to review the current legal minimum catch size of the species under study as established by Resolution 0595 of 1978 (20.0 cm LS), because these results suggest that the minimum legal catch size does not correspond to Liseta biology, and therefore updating the existing regulation would improve fishery management and conservation in the wild.
As far as the average diameter of mature oocytes estimated in this study (977 µ), it was observed that it is the lowest of all those reported in South America. In Argentina, Leporinus obtusidens reaches 1100 µ in the Upper River Paraná 13. In Brazil, Leporellus vittatus presents 1212 µ, Schizodon nasutus 1232 µ, Leporinus lacustris 1259 µ and L. friderici 1060 µ, also in the Upper Paraná River 7. In captivity, L. muyscorum in Colombia has an observed mean diameter of 929 µ 18, while L. macrocephalus in Brazil is 1215 µ 19. These differences can be associated with each species’ fecundity.
The reproductive period of rheophilic fish in the Sinú River is at the start of the rainy season, so the first spawning may occur between March and April if the necessary changes in water flow and physical and chemical states occur. This was confirmed in this study, where individuals with sexually mature ovaries and testes were collected during April and May (rising waters), females with mature ovaries in August, and males with mature testes in July (high water) and males with mature testes in September (low water). In addition, fish were also collected in a state of advanced sexual maturity in February (low water), which allows us to infer that the new hydrological dynamics present in the Sinú River since 2000 could be influencing the species’ reproductive cycle, which has been observed for other rheophilic fish like Blanquillo Sorubim cuspicaudus and Dorada Brycon sinuensis.
However, it was reported that capture of very small and very young individuals has caused overfishing in recruitment and growth, indicating that the species is going through a critical time. Entities that manage fishing in the Sinú basin should take this into account and make necessary medium-term changes 3. This situation is aggravated during spawning season because the capture of rheophilic fish, especially Characiform, increases considerably when they migrate short distances upstream or downstream, as in the case of the Sinú River.
In addition, disrupting fish migration to maturation and spawning areas upstream of the Urra hydroelectric plant, spawning loss upstream of the dam, altered water quality in maturation and spawning areas downstream from the tunnels, and overfishing of rheophilic fish that have the ability to reproduce result in the loss of reproductive potential, all as a result of the construction of the Urra hydroelectric plant. For these reasons, Liseta has been classified as vulnerable nationwide, highlighting the need for programs to protect habitat and fishery regulations to conserve the species 20.
Fecundity is a specific reaction to changing conditions, particularly mortality, which fluctuates in response to food supply, and is a basic mechanism to adjust reproduction rates to environmental changes. These changes are reflected in different fecundity rates between populations and species 21 and are affected by the size and weight of the fish, ovary weight, and the size of mature oocytes. Estimated fecundity in this study (30793 oocytes) is much lower than that reported for this species in Colombia through induced breeding (41484 oocytes) 18, and different from estimates by different authors for the Anostomidae family 7,22, although similar to that estimated for Leporellus vittatus (34600) 7, as presented in table 3.
Table 3 Fecundity reported for the Anostomidae family in South America.
| Species | Fecundity (Num. of oocytes) | Source |
|---|---|---|
| Leporellus vittatus | 34600 | Vazzoler (7), 1996 |
| Leporinus friderici | 194000 | Vazzoler (7), 1996 |
| Leporinus muyscorum | 41484 | Argüello et al (18), 2001 |
| Schizodon nasutus | 77000 | Vazzoler (7), 1996 |
| Schizodon knerii | 88500 | Sato et al (22), 2003 |
| Leporinus muyscorum | 30793 | This study |
The results allow us to conclude that Liseta is a fish that has a F:M sex ratio of 2.6:1 with annual spawning and synchronous oocyte development in two seasons that extend from February to September, with large oocytes and high fecundity that are closely associated with the weight of the ovaries.