INTRODUCTION
The mycobacteriosis is the common occurrence in many fish species, involvement of more than one species of Mycobacterium in fish infections, ranging manifestation of disease, although typically including granuloma formation 1 with potential for zoonotic transmission 2.
It is characterized by the formation of a granuloma with typical acid-alcohol resistant bacillus (BAAR) after staining with Ziehl-Neelsen 3. Due to its infectivity, the lack of details for host selection and environmental ubiquity including water, soil, dust, aerosol, and from animals 4. Mycobacteriosis is an emerging disease and also one of the most important chronic infectious diseases in the aquaculture/ornamental fish 5.
The disease develops from the chronic situation until the formation of a granuloma, it is associated with low mortality and can remain asymptomatic for a long time, causing damages on fish growth that become non marketable, and in acute cases all fish can be lost 6.
The granuloma is a focal compact collection of inflammatory cells with predominance of mononuclear cells and is generally formed as a result of a persistence of some product non degradable with active hypersensitivity 7,8. This formation consists in a final result of a complex interaction among the invader organism/antigen, chemical products, drugs or other irritants, macrophage activity, response of Th1 helper cells, hyperactivity of B cells, circulating immune complexes and chemical and biological mediators 3,4. Inflamed or immunological reactivity areas recruit monocytes in the blood 9 and these cells differentiate to macrophages after diapedesis 10 which merge to generate the multinucleated giant cells at site of inflammation, or differentiate in secreting macrophages known as epithelioid type cells 8,11-13.
Mycobacteria have the capacity of delude the host immune defense 14,15 and to survive inside the macrophages. Additionally, they can also present zoonotic risk 16. The use of Bacillus Calmette-Guérin (BCG) is the unique vaccine against tuberculosis being one of the most used in the last 100 years worldwide 17.
Studies demonstrate that its use induce to a granuloma formation 8,18 but is not constant and depends on the phylogenetic position of fish 19 also influenced by a great variety of fishes 20 in which becomes few clear the events involved in the process until the granuloma formation. Pacu (Piaractus mesopotamicus) is a neotropical freshwater species of great importance in South America finfish aquaculture, and this teleost fish has demonstrated to be a good bioindicator of water quality and has been used as model for ecotoxicity studies 21,22. In this context, this study evaluated the cell kinetic and formation of granuloma during chronic inflammation induced by BCG in the skeletal muscle of pacus, as a model to study innate immunity.
MATERIAL AND METHODS
Animals and conditioning. Sixty healthy juvenile of pacus with mean weight 120±5 g were randomly distributed in ten 250 L plastic tanks with water supplied from an artesian well at a flow of 1 L/min with supplementary aeration. They were fed commercial feed (3% of the biomass, 28% of GP and 4000 kcal of GE/kg). The parameters physical and chemical of the water were measured with a probe YSI Model MPS 556.
Experimental design. After acclimatized for seven days the fish were randomly distributed in two groups (BCG-inoculated and non-inoculated) 30 fish in each group. To evaluate the inflammatory process, five subgroups of six fish from each group were collected for analysis at 3, 7, 14, 21 and 33 days post-inoculation (DPI).
Induction of the inflammatory process. To the inoculum of BCG, the fish were anesthetized by immersion in an aqueous solution of benzocaine (1:10000 v/v) prediluited in alcohol (0.1 g/mL) and inoculated with 40 μL of 2.2 x 106 colony forming unities (CFU)/mL of inactivated bacilli (BCG from Rio de Janeiro) using a syringe BD ULTRA-FINE™. The inoculum was made on the muscle tissue in the laterodorsal region, equidistant between the start of the dorsal fin and the midline. After procedure, they returned to their tanks, with continuous water flow and constant aeration. Non-inoculated fish were exposed to the same procedures but did not receive inoculation (Ethics Committee CEUA-UNESP protocol nº 020092/09).
Histopathology. After euthanasia in a benzocaine solution (1:500 v/v), fragments of the inoculated muscle were collected, fixed in formalin solution 10% buffered for posterior routine histological techniques and embedded in paraffin. Cross sections of 5 μm thickness were stained with hematoxylin-eosin (HE) for histopathological examination and Ziehl-Neelsen (ZN) reagent to identify the bacillus. The slides were examined under microscope Olympus (BX 51) and the images captured with digital camera (Olympus DP73).
RESULTS
Histopathological analysis of HE stained slides of non-inoculated fish showed no signs of chronic inflammation as also confirmed after Ziehl-Neelsen staining. On the other hand, BCG-inoculated fish showed the bacilli presence (Figure 1). For evolution process study, inoculated fish showed a diffuse inflammatory reaction in the muscle 3 DPI mostly composed by mononuclear cells with dense chromatin and reduced cytoplasm (Figure 2-a). There were also observed multinucleated giant cells with phagocytic activity, some plasmocytes cells and pigments that might be associated with erythrocyte lysis, besides interstitial edema characterized by dissociation of the muscle fibers (Figure 2-b).
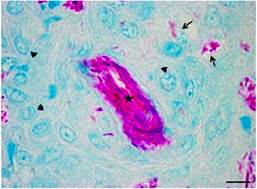
Figure 1 Photomicrograph of the muscle tissue of Piaractus mesopotamicus, fourteen days after inoculation of BCG. Can be seen macrophages (arrowhead) arranged around the inoculum (star), and phagocyted bacilli (large arrow). (ZN). Scale bar = 6 µm.
The inflammation continued diffuse 7 DPI initiating the cellular organization surrounding the inoculum (Figure 2-c). Mononuclear infiltrate was greater with the formation of the multinucleated giant cells, persistence of the interstitial edema and tissue necrosis. In the blood vessels the presence of mononuclear cells disposed close to the axial stream blood was observed besides foci of perivascular cell infiltrate containing dense chromatin nuclei and few cytoplasm. In the inflammatory infiltrate, brownish granules compatible with hemosiderin possibly derived from hemorrhage (Figure 2-d) was evidenced. Surrounding the inoculum fibroblast-like cells was found.
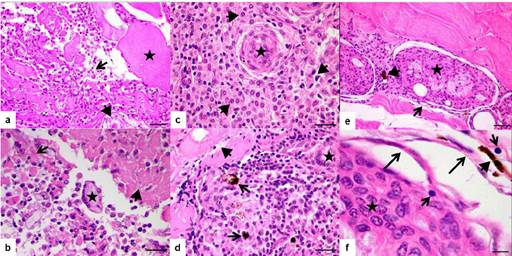
Figure 2 Photomicrographs of the muscle tissue Piaractus mesopotamicus. a) Three days post-inoculation of BCG can be seen cellular infiltrated (large arrow), interstitial edema (arrowhead), muscle fibers (star). (HE). Scale bar = 30 µm. b) Three days after inoculation of BCG can be seen multinucleated giant cells (star), tissue necrosis (arrowhead), interstitial edema (large arrow). (HE). Scale bar = 20 µm. c) Seven days post-inoculation of BCG can be seen the beginning of granuloma formation with macrophages (star) and mononuclear cell infiltrate (arrowhead) (HE). Scale bar = 20 µm. d) Seven days post-inoculation of BCG can be seen melanomacrophages (large arrow), multinucleated giant cells (star), edema (short arrow). (HE). Scale bar = 20 µm. e) Fourteen days post-inoculation of BCG can be seen granuloma formation with central necrosis (star), presence of fibroblasts (large arrow), melanomacrophages (arrowhead). (HE). Scale bar = 30 µm. f) Fourteen days post-inoculation of BCG can be seen granuloma formation with macrophages and epithelioid type cells (star), fibroblasts (large arrow), lymphocyte (short arrow), and melanomacrophages (arrowhead). (HE). Scale bar = 6 µm.
Interstitial edema with discrete presence of the multinucleated giant cells was found 14 DPI. The foci of necrosis were not more visualized; the inflammatory focus was more organized with the presence of mononuclear infiltrate, pigment debris similar to hemosiderin indicating erythrocyte lysis and fibroblasts surrounding the inoculum. It was observed typical morphology of a granuloma with a central inoculum besides mononuclear cells infiltration in the peripheral region (Figure 2-e). Discrete presence of epithelioid-like type cells with acidophilic cytoplasm and floppy chromatin with the presence of some lymphocytes and great amount of fibroblasts was observed (Figure 2-f). It can be observed higher cellular organization surrounding the granuloma, intense peripheral mononuclear inflammatory infiltrate with the presence of hemosiderin pigments at 21 DPI with increase in the number of fibroblasts and macrophages cell surrounding the inoculum (Figure 3-a and 3-b).
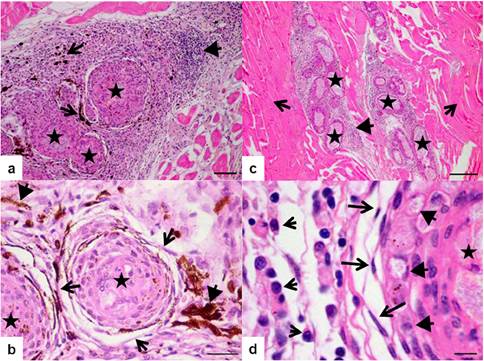
Figure 3 Photomicrographs of the muscle tissue Piaractus mesopotamicus. a) Twenty one days post-inoculation of BCG can be seen granuloma formation (star), mononuclear infiltrate (arrowhead), pigmentation associated with the presence of melanomacrophages (large arrow) (HE). Scale bar = 60 µm. b) Twenty one days post-inoculation of BCG can be seen granuloma formation (star) with fibroblasts (arrowhead) and pigments of hemosiderin (short arrow) (HE). Scale bar = 20 µm. c) Thirty three days post-inoculation of BCG can be seen granuloma formation (star) mononuclear infiltrate (arrowhead) and muscle fibers (large arrow) (HE). Scale bar = 200 µm. d) Thirty three days post-inoculation of BCG can be seen the inoculum in the center of the granuloma (star), epithelioid type cells (arrowhead), fibroblast surrounding the macrophages layer (large arrow), macrophages (short arrow). (HE). Scale bar = 6 µm.
At 33 DPI, the inflammatory process became less diffuse even presenting mononuclear infiltrate, formation of small amount of granuloma surrounded by the same type of reaction found in bigger granuloma (Figure 3-c). Both the young and old granuloma presented typical characteristic around the inoculum composed by a layer of epithelioid-like cells, besides macrophages, some lymphocytes and abundant fibroblasts (Figure 3-d).
DISCUSSION
The inflammatory process in pacus has initiated with intense perivascular reaction composed mostly by macrophages that had migrated to the lesioned site, similar to that observed by Sado and Matushima 18. Over the course of time, it formed multinucleated giant cells as also described in P. mesopotamicus by Manrique et al 8. Contrarily, Ishikawa 23 did not observe giant cells 84 DPI with Mycobacterium marinum in Oreochromis niloticus. This difference could be in part attributed to the different bacterium strain used and fish species.
Epithelioid type cells were also found by Manrique et al 8 studying the granuloma formation in P. mesopotamicus, and these cells started to be observed in the 14th day of chronic granulomatous inflammation, confirmed by immunohistochemical staining. The great number of melanomacrophages observed in this study might be strongly related to the hemorrhage present in the muscular lesion. Consequently, these cells have phagocytosed the hemosiderin as a result of erythrocyte lysis, as described by Ramos et al 24 in visceral granuloma naturally diagnosed in cultured Sparus aurata. During granulomatous inflammation increased number and size of melanomacrophages were observed in the spleen of O. niloticus25.
The granuloma formation was evident with organized macrophages disposed around the inoculum, epithelioid type cells and foreign body like giant cells at 33 DPI, corroborating previous studies in Centropomus spp. (18). Nevertheless, it was not found the formation of caseous center contrarily to that found in Paralichthys orbignyanus and Elacatinus figaro naturally infected by M. marinum26.
Cellular staining of the granuloma herein observed developed according to Gauthier et al 27 description in Morone saxatilis experimentaly infected with M. marinum where the macrophages with phagocytic activity have suffered morphophysiological changes and became transformed in epithelioid type cells with secreting activity. This feature will be maintained until the immune like granuloma as described by Mariano 28. In conclusion, this study showed the feasibility of using the P. mesopotamicus to study chronic granulomatous inflammatory response induced by BCG, characterized by changes in the kinetics of inflammatory cells in skeletal muscle classifying as immune-epithelioid type, similar to granulomatous inflammation caused by M. marinum.














