INTRODUCTION
Canine Visceral Leishmaniasis (CVL), a well recognized parasitic and zoonotic disease caused by an intracellular protozoan of the genus Leishmania1, is transmitted to the canine species by the bite of blood-sucking sand flies (Phlebotomusspecies). The disease might exist subclinically or might manifest as a systemic involvement [cutaneous, ocular or renal lesions, hepatosplenomegaly, anemia, muscular atrophy and weight loss] 2. Within the last 15 years clinical signs of cardiac disease are reported in relationship with leishmaniasis 3-6, myocardial lesions might exist 3-7, thus the parasite could be detected in cardiac tissue 3,4,6.
Thus, the goals of this study composed of dogs naturally exposed to CVL were to (1) quantify the echocardiographic and electrocardiographic findings and alterations in cTnI concentrations, 2 determine if different stages of CVL is related to cardiac injury and 3 evaluate the association of hematologic, serologic and serum biochemical abnormalities and severity of disease (different stages of CVL).
MATERIALS AND METHODS
Site of the study and animal population. The study was performed at the University of Adnan Menderes, Faculty of Veterinary, Department of Internal Medicine (37.828922 latitude and 27.793518 longitude) located in Eagean region of Turkey) to those of dogs referred to clinics with disease associated signs.
Grouping and classification of animals. Prior to study necessary ethical guidelines were taken into consideration, and a written owner consent was available for all dogs involved. A total of 35 dogs, comprising 28 with CVL and 7 healthy were enrolled. Diagnosis of CVL was established through dogs presenting one or some of the clinical signs in association with the disease condition, subjected to rapid ELISA based test kits and immune fluorescence antibody test. Dogs diagnosed with CVL, based on serological, clinical, hematological and biochemical findings, were classified into 4 different groups (n=7), as reported by Leishvet Group 8. Aforementioned research groups were assigned in a subset of five major groups of dogs studied were as follows;
I group: Stage I (mild disease)
II group: Stage II (moderate disease)
III group: Stage III (severe disease)
IV group: Stage IV (very severe disease)
V group: Healthy controls.
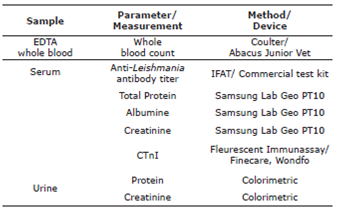
Laboratory analysis deemed necessary for staging of the diseases were shown in table 1.
Hematological examination. Blood samples were witdrawn from Vena cephalica antebrachii. A total of 2.5 mL anticoagulated (EDTA) blood was forwarded to laboratory for complete blood counts by use of automatic cell counter (Abacus Junior). Leukocyte (WBC), erytrocyte (RBC), packed cell volume (PCV), hemoglobine (HGB), mean erytrocyte volume (MCV) and mean erytrocyte hemoglobin concentration (MCHC) and thrombocyte (PLT) values were detected.
Urinalysis. Voided urine sample or urine collected by catheterization (5 mL) were analyzed for total protein and creatinine concentrations in a private [Bio-Rad Eqas, Vetqas (Veterinary Laboratory Quality Assurance Unit) and Vqas (Veterinary Laboratory Quality Association Assurance Programme)] outer quality programme partnership laboratory (Bilim Special Veterinary Diagnosis and Analysis Laboratory, Istanbul, Turkey), in which urinary protein/creatinine ratio was calculated colorimetrically. All urinary samples were forwarded immediately under cold packages.
Serology-Based Assays. ELISA based test kits were used according to the manufacturer’s instructions to detect Leishmania infantum (Snap Leishmania test kit, IDEXX) and to those of E. canis/E. ewingii, A. phagocytophilum/A. platys, Borrelia burgdorferi antibodies and Dirofilaria immitis antigens (Snap 4Dx plus, IDEXX). An immunofluorescence assay 8,9 was used to test canine sera for antibodies againstLeishmania infantum (Leishmania IgG) as previously described 10. Serum aliquots for serology tests were stored until analysis. The IFAT result deemed positive for CVL with a 1:64 and above dilution of the serum gave an evident yellow-green fluorescent signal upon microscopical detection, whereas nonreactive (without samples yellow-green fluorescent) appeared negative 10.
Serum cTnI concentrations. Serum cTnI concentrations were determined at the Department of Internal Medicine, Faculty of Veterinary, University of Adnan Menderes, with a commercial troponin I analysis system (Wondfo Finecare Fluorescent Immunoassay) previously validated. Serum aliquots were stored until study, and were thawed just prior to the moment of the analysis. Measurements below the lower limit of detection (0.1 ng/mL) were assigned this value for the statistical analysis. Linear range (min-max) of the Finecare Fluorescent Immunoassay was 0.1-50 ng/mL.
Serum biochemical analysis. Total Protein, albumine and creatinine levels were determined by use of chemistry analyzer (Samsung Lab Geo PT10; Korea).
Cardiac evaluation. Cardiac examination was performed [electrocardiographic (ECG), echocardiographic (ECHO) and cnTI analysis] in dogs infected with leishmaniasis [in an attempt to diagnose, detect presence and severity of cardiac injury] and to those of healthy dogs [for comperative evalaution].
For ECG examination standard 10-lead ECGs (leads I, II, III, aVR, aVL, aVF, rV2, V2, V4, and V10) were recorded at least for 60 seconds in all cases by use of a computer-based ECG monitore with a speed of 50 mm/s, calibrated for 10 mV/cm. ECGs were analyzed for heart rhythm and rate, alterations in amplitude of T wave/ST segment, conduction disturbances. ECGs were rated according to severity of abnormalities, as previously described 11.
ECHO analysis were performed within the right parasternal sagittal plane in a motion (M) mode with an electronic 2.7-5 MHz probe, with portable ECHO machine (Mindray M5, China) as purchased within the budget of this project. Parameters such as left ventricle internal diameter in diastole (LVIDd) and systole (LVIDs), fractional shortening (FS) and ejection fraction (EF) [using Teichholz method] were calculated 12,13. From every case enrolled, four consecutive heart cycles were averaged involving each parameter 14. Left ventricular systolic dysfunction was defined as a fractional shortening of 26 % 14.
Statistical analysis. Statistical analysis of the results was performed by use of SPSS 18.0 for Windows (SPSS, 2009). In each group during sampling time arithmetic mean (X), standard deviation (s) and minimum-maximum (Xmin-Xmax) values were calculated. Given the set of data, the present researchers checked if its distribution was normal. Two tests, Shapiro-Wilk or Kolmogorov-Smirnov, were run for normality. Parameters not normally distributed were subjected to nonparametric methods. Comparison of parameters in more than 2 groups were performed by Kruskall-Wallis test, post-hoc pairwise comparisons
RESULTS
Signalment. Dogs of 11 different breeds were enrolled, as shown in table 2. Twenty three dogs were purebred, whereas 12 were of crosbred. Enrolled dog population consisted of 26 males and 9 females, distributed as following: Group I dogs: 3 males and 4 females; Group II: all male; Group III and IV each 6 males and 1 female and controls: 4 males and 3 females. The body weight ranged from 7 to 41kg and did not vary significantly among the groups.
Table 2 Demographic information of infected cases and control group
| Stage | Case | Sex | Breed | Age (Year) | Weight (Kg) | |
|---|---|---|---|---|---|---|
| I | 1 | M | Crossbred | 2 | 28 | |
| 2 | F | Spaniel Cooker | 1 | 8 | ||
| 3 | M | Crossbred | 2 | 18 | ||
| 4 | F | Golden retriever | 3 | 24 | ||
| 5 | F | Crossbred | 4 | 12 | ||
| 6 | F | Labrador Retriever | 1 | 16 | ||
| 7 | M | Crossbred | 3 | 11 | ||
| II | 1 | M | Pointer | 3 | 20 | |
| 2 | M | English mastiff | 6 | 38 | ||
| 3 | M | German shepherd | 2 | 23 | ||
| 4 | M | Crossbred | 1 | 15 | ||
| 5 | M | Crossbred | 1 | 16 | ||
| 6 | M | Terrier | 2 | 9 | ||
| 7 | M | Crossbred | 4 | 22 | ||
| III | 1 | M | Dogo Argentino | 5 | 41 | |
| 2 | M | Kangal | 1,5 | 35 | ||
| 3 | M | Terrier | 4 | 7 | ||
| 4 | M | Presa Canario | 5 | 51 | ||
| 5 | M | Boxer | 4 | 18 | ||
| 6 | F | Crossbred | 12 | 13 | ||
| 7 | M | Dogo Argentino | 3 | 32 | ||
| IV | 1 | M | Dogo Argentino | 3 | 38 | |
| 2 | M | Spaniel cocker | 6 | 28 | ||
| 3 | M | Boxer | 4 | 16 | ||
| 4 | F | Golden retriever | 4 | 20 | ||
| 5 | M | Cooker | 3 | 25 | ||
| 6 | M | Kangal | 2,5 | 25 | ||
| 7 | M | Golden retriever | 14 | 28 | ||
| 1 | M | Crossbred | 3 | 10 | ||
| 2 | M | Terrier | 4 | 14 | ||
| 3 | F | Dogo argentino | 1 | 16 | ||
| Control | 4 | M | Crossbred | 2 | 18 | |
| 5 | F | Kangal | 6 | 15 | ||
| 6 | F | Crossbred | 2 | 15 | ||
| 7 | M | Crossbred | 8 | 18 | ||
| M= Male, F= Female |
Clinical findings. Control group of dogs were subjected to physical examination and laboratory analysis aforementioned above and suggested as healthy. Based on laboratory parameters (Table 1) and staged according to Leishvet guidelines, diseased groups presented clinical signs as shown in table 3.
Table 3 Number of cases showing clinical manifestations in infected groups
| Symptoms | Groups | ||||
| I | II | III | IV | V | |
| High body temperature | 0 | 0 | 1 | 2 | 0 |
| Lymphadenopathy | 7 | 7 | 7 | 7 | 0 |
| Weight loss | 3 | 5 | 7 | 7 | 0 |
| Onychogripozis | 2 | 3 | 3 | 4 | 0 |
| Hypotrichosis | 7 | 7 | 7 | 7 | 0 |
| Periocular alopecia | 0 | 1 | 1 | 1 | 0 |
| Skin lesions | 7 | 7 | 7 | 7 | 0 |
| Epistaxis | 0 | 0 | 0 | 2 | 0 |
IFAT titers. The IFAT was positive for all dogs in the infected groups. IFAT titers according to groups and individual cases were shown in table 4.
Table 4 IFAT analysis results of groups
| Group | I | II | III | IV | V | ||||||||
| Case | All | 1 | 2 3 4 5 6 7 | 1 | 2 3 | 4 5 6 | 7 | 1 | 2 3 4 | 5 | 6 | 7 | All |
| İFAT | 1/ 64 | 1/ 512 | 1/ 128 | 1/ 512 | 1/ 128 | 1/ 256 | 1/ 512 | 1/ 1024 | 1/ 2048 | 1/ 4096 | 1/ 8000 | 1/ 16000 | 0 |
Cardiac Troponin I results. Among control group and to those of groups I., II. and IV. there was no alteration in CTnI concentrations, all values were detected under the lower limits of detection (<0.1 ng/mL), whereas solely in one dog (in G III) presented midly elevation (0.16 ng/mL).
Hematological results. Hematological data and relevant descriptive statistics were presented in table 5. There were statistical significance regarding mean (±standard deviation) values for WBC [among healthy control group (V.) and other groups (p=0.049)], RBC [among stage III-stage IV and other groups (p=0.001)], Hb [between stage I and stage III-stage IV (p=0.008), HCT [between stage I and other groups (p=0.001)] and MCHC [between stage I and stage III- stage IV (p=0.046)].
Table 5 Descriptive statistics of relevant hematological data for CVL infected dogs
| Data | Groups | |||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | P value | |
| WBC (x109/L) | 15.8±4.9b (10.7-22.1) | 17.2±7.4b (10.2-31.6) | 21.2±17.9b (5.8-59) | 16.4±9.4b (7.6-26.9) | 7.6±1.2a (6.2-9.4) | 0.049 |
| RBC (x1012/L) | 5.8±0.5a (5.1-6.9) | 5.8±1.7a (4.1-9.2) | 4.4±0.6b (3.7-5.2) | 4.8±1b (3.95-6.1) | 7.2±0.7a (6.2-7.9) | 0.001 |
| HGB (g/dL) | 13.8±1.5a (11.0-16.3) | 13.5±4.3a.b (8.4-21.8) | 9.9±2.2b (7.1-13.5) | 10.9±2.9b (8.3-15.6) | 14.9±1.4a (12.2-16.8) | 0.008 |
| HCT (%) | 37.2±3.8a (30.6-43.2) | 37.0±10.8b (25.3-57.1) | 28.9±4.5b (23.0-35.5) | 31.2±7.0b (23.9-39.8) | 43.7±1.8a (41.0-46.1) | 0.001 |
| MCV (fL) | 63.7±2.3 (60.0-66.0) | 63.4±2.5 (61.0-68.0) | 65.4±3.8 (61.0-72.0) | 64.857±2.9 (61.0-70.0) | 66.0±2.1 (63.0-69.0) | 0.359 |
| MCHC (g/dL) | 37.0±1.1b (35.7-38.3) | 36.2±1.8a.b (33.0-37.9) | 33.8±3.5a (30.9-38.3) | 34.5±2.5a (31.1-39.2) | 34.3±1.5a (32.2-36.5) | 0.046 |
| PLT (x109/L) | 437.5±204 (202-681) | 334.8±287 (32-717) | 249.2±45.3 (185-316) | 185.1±97.5 (64-317) | 318.8±62.6 (248-401) | 0.121 |
| Mean ± standard deviation (minimum-maximum), Difference between groups according to letters a and b are shown | WBC: White blood cell, RBC: Red blood cell, HGB: Hemoglobin, HCT: Hematocrit, MCV: Erythrocyte mean volume, MCHC: Mean hemoglobin volume in erythrocytes, PLT: Thrombocyte | |||||
Serum biochemical results. Among dogs infected with Leishmaniasis serum biochemical analysis (albumine, creatinine and total protein) performed for staging of the disease with descriptive statistics were denoted on table 6. Considering serum biochemical findings there were significant differences for serum creatinine [(p=0.008) among G IV and other groups, except G III], serum TP [(p=0.002) among G IV and other groups, except for G II], serum albumine [(p=0.004) among stage IV and stage I-II].
Table 6 Albumine, creatinine and total protein values of CVL-infected and control dogs according to groups
| Groups | ||||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | p value | |
| ALB (g/dL) | 2.52±0.31a (2-2.86) | 2.3±0.43a (1.84-3.16) | 2.1±0.42a,b (1.3-2.76) | 1.71±0.28b (1.3-2.1) | 2.21±1.2a,b (2-2.5) | 0.004 |
| CREA (mg/dL) | 0.78±0.27a (0.44-1.3) | 0.79±0.3a (0.48-1.3) | 1.03±0.59a,b (0.6-2.3) | 1.65±0.97b (0.6-2.6) | 0.56±0.16a (0.4-0.8) | 0.008 |
| TP (g/dL) | 5.7±1.76a (2.8-8.3) | 6.0±0.62a,b (5,4-6,8) | 5.4±0.85a (3.86-6.22) | 7.6±1.25b (5.5-8.9) | 4.67±0.9a (3.6-5.8) | 0.002 |
| ALB: Albumine, CREA: Creatinine, TP: Total protein. Mean ± standart deviation (minimum-maximum). The differences between the groups according to letters a and b are shown. | ||||||
Electrocardiographic examination. To those of enrolled dogs, ECG examination was performed once in CVL infected and control groups, as shown in table 7. Two of the present researchers (Prof. Dr. K.U. and DVM C.B.), who were well experienced for many years in ECG and ECHO examination, performed analysis. First author spent 17 years in cardiology field, and participated within the ECG and ECHO examinations of several cases. Moderate or severe ECG abnormalities were detected in 6/28 of diseased dogs (Table 7); however, with 3 out of 7 cases in stage IV dogs, showing evidence that as the disease status progress, more dogs presented ECG abnormality, at least for this study. Control dogs were also included within the comparison, altough these dogs were selected for the absence of ECG abnormalities. Some of the dogs enrolled in groups presented more than 1 ECG abnormality, to those of which mild sinus tachycardia (n=4), high T waves (n=5) and ST segment elevation (n=5, with 3 cases in stage IV) were prominently evident.
Table 7 Electrocardiographic findings* among dogs infected with leishmaniasis and to those of control group (V) of dogs
| ECG Abnormality* | Group | ||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| Mild sinus tachycardia | 1 | 1 | 1 | 1 | 0 |
| Persistent sinus tachycardia | 0 | 0 | 1 | 1 | 0 |
| Bradycardia | 0 | 0 | 0 | 1 | 0 |
| Atrial premature complex | 0 | 1 | 0 | 0 | 0 |
| High T waves | 0 | 0 | 2 | 3 | 0 |
| ST segment elevation | 0 | 0 | 2 | 3 | 0 |
| ECG abnormalities by severity | |||||
| No abnormality or mild | 7 | 6 | 5 | 4 | 7 |
| Moderate | 0 | 1 | 1 | 2 | 0 |
| Severe | 0 | 0 | 1 | 1 | 0 |
| *Schober et al (11). | |||||
Echocardiographic examination results. Regarding ECHO examination of infected groups of dogs with CVL (I.-IV.) mean (± standard deviation) LA/Ao value presented significant difference (p=0.003) among stage IV and other groups. Although there were insignificant individual differences regarding other parameters (FS, EF, LVIDs and LVIDd), overall evaluation did not present differences among groups which was shown in table 8 and 9.
Table 8 Evaluation of ECHO findings*
| ECHO Abnormalities* | Groups | ||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| Decreased FS * | 0 | 1 | 2 | 3 | 0 |
| Decreased LVIDS ** | 1 | 3 | 2 | 5 | 0 |
| Decreased LVIDd ** | 1 | 2 | 3 | 4 | 0 |
| Enlarged atrium (1.5<LA/Ao<2)*** | 1 | 0 | 2 | 2 | 0 |
| Significantly enlarged atrium (LA/Ao>2)** | 0 | 0 | 0 | 1 | 0 |
| *Diniz et al (14) **Categorical assessments were based on normal reference values for dogs and predicting intervals for variable weights (body weight); Cornell et al (26) *** Tai et al (29). | |||||
Table 9 Statistical evaluation of ECHO findings
| Data | Groups | p value | ||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Weight | 17.4±7.6 (8-28) | 19.7±9.1 (9-38) | 29.4±15.2 (7-51) | 23.7±4.8 (16-28) | 15.1±2.7 (10-18) | 0.100 |
| LVIDs | 2.1±0.5 (1.4-2.9) | 1.7±0.5 (1.0-2.2) | 2.3±0.9 (0.9-3.2) | 2.0±0.7 (1.5-3.1) | 2.23±0.6 (1.8-3.2) | 0.443 |
| LVIDd | 3.3±0.6 (2.3-4.2) | 3.2±0.4 (2,7-4,0) | 3.7±1.0 (2.5-5.0) | 3.5±0.6 (2.9-4.2) | 3.2±0.7 (2.5-4.2) | 0.777 |
| EF | 67.4±6.1 (59-74) | 76.6±14.4 (58-93) | 64.6±19.2 (35.3-93.8) | 73±14 (52-82.6) | 60.6±9.3 (48-74) | 0.200 |
| FS | 36.6±4.4 (31-42) | 46.4±14.4 (29-65) | 37.2±16.2 (16.4-65.4) | 37.6±11.2 (26.9-50) | 32.3±6.8 (24-42) | 0.635 |
| LA/Ao | 1.3±0.3a (0.9-1.7) | 1.1±0.2a (0.7-1.3) | 1.3±0.2a (1-1.6) | 1.8±0.2b (1.5-2.1) | 1.3±0.2a (1.1-1.5) | 0.003 |
| Mean ± standart deviation (minimum-maximum) The differences between the groups according to letters a and b are shown. | ||||||
Urinalysis. UPC analysis revealed control group values were <0.1. On the other hand UPC values were <0.1-0.3 in G I, 0.5-1 in G II, 2-3 in G III, 5-10 in G IV. All values were shown in table 10. Statistical difference was evident among control group and stage II to IV dogs ((p=0.000).
Table 10 Values and statistical evaluation of the UPC analysis
| Data | Groups | p value | ||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| UPC | 0.15 ±0.1a,b (0.1-0.3) | 2.4 ±0.5b (2.0-3.0) | 2.4 ±0.5b (2.0-3.0) | 6.9 ±2.2b (5.0-10.0) | 0.09 ±0.0a (0.09-0.09) | 0,000 |
| Mean ± standart deviation (minimum-maximum) The differences between the groups according to letters a and b are shown. | ||||||
DISCUSSION
The present study was conducted with the financial and scientific (3001 Tübitak Project) support of The Scientific and Technological Research Council of Turkey, in which updated staging of the disease was taken into consideration based on the crietaria by Leishvet Guidelines 8. Therefore it should not be unwise to draw conclusion that cardiological examination based on ECG and ECHO findings and cnTI analysis according to the stages of CVL were not reported previously.
Taking into account Leishvet Guidelines serological (IFAT titers and additional ELISA tests), clinical findings and laboratory evaluation (TP, Alb and creatinine) dogs enrolled in this study were participated in 4 different infected groups (stage I to IV). Stage I included (low positive antibody titers:1/64), to stage IV (high positive antibody levels: 1/1024-1/16000). In agreement with the proposed staging and the relevant literature (8), stage I and II (to those of groups denoted with same numbers) revealed normal renal profile, whereas stage III dogs presented chronic kidney disease (CKD) IRIS stage I or stage II, and finally stage IV dogs showed CKD) IRIS stage III or stage IV.
Regarding hematological data statistical significance were evident for WBC [among healthy control group (V.) and other groups (p=0.049)], RBC [among stage III-stage IV and other groups (p=0.001)], Hb [between stage I and stage III-stage IV (p=0.008), HCT [between stage I and other groups (p=0.001)] and MCHC [between stage I and stage III- stage IV (p=0.046)]. As the disease progress erythrocyte markers altered, as expected.
Cardiac troponins (cTn) are used as blood biomarkers with high specificity and sensitivity for detecting myocardial degeneration. The latter contractile proteins, might be released from myocardium in relation with the severity of tissue injury and myocyte membrane disruption 15. Speficifically detectable elevations in circulating cTnI were reported in Canine Monocytic Ehrlichiosis 14. Regarding the relation between cTnI and leishmaniasis limited portion of study took place in the literature. Detailed literature search revealed case reports 16, and some limited original studies 17,18.
A recent study reported that myocarditis in CVL 3,5 could be in association within immunological alterations due to Leishmania infection 7. Another study inspected the hearts of 30 dogs naturally infected with Leishmania infantum chagasi, detected myocardial lesions in all dogs, and the parasite was found in the cardiac tissue (20/30 dogs). According to the results of that study cardiac lesions might be prevalent in dogs with naturally occurring CVL even in the absence of clinical signs related to heart failure 6.
A prior study was the subject of an evaluation for serum cTnI as an indicator of myocardial injury in dogs with leishmaniasis 17. In that study in 40 dogs with leishmaniasis, median cTnI concentration was significantly (p =0.011) higher in contrast to 11 control dogs. Sixteen dogs (40%) with CVL presented elevated cTnI concentration. There was moderately to weak correlation among cTnI with decreased positive Leishmania titer, and increased age, whereas cTnI concentration did not differ between azotemic animals and control dogs 17. However serum cTnI concentration might have interaction with age, thus could be elevated with marked azotemia, a finding frequently associated with canine leishmaniasis 15. In the present study there was no increase on serum cTnI concentrations among dogs with different stages of CVL infected dogs, altough advanced stages (stage III or IV) caused chronic renal failure.
A total of 30 non-uremic dogs with leishmaniasis in Greece, were prospectively enrolled as 20 in Group A were treated with a combination of meglumine antimonate and allopurinol for 28 days, whereas 10 dogs participated in Group B were treated with allopurinol alone 18. Blood samples were collected at timepoint 0 (before treatment) and at 14 and 28 days after the initiation of treatment. None of the dogs treated with meglumine antimonate in group A presented a serum cTnI concentration above the upper limit of the reference range (>0.5 ng/mL) nor cardiotoxicity at 2 and 4 weeks after the initial therapy 18.
As aforementioned above limited literature evaluated intravital diagnosis of cardiac alterations in leishmaniasis. Cardiac involvement in dogs 3,6,7,17 revealed that this protozoan might be also involved in heart, which promptly support the necessity of cardiac evaluation in cases of suspected leishmaniasis.
In the present study except 1 case, cnTI levels were not changed. Although it is not possible to make interpretation solely analyzing cnTI levels, it was an interesting and unexpected finding. Repeated measurement was not available, excluding out to interpret persistent elevation. Persistent elevation in cTnI levels suggested irreversible and active continuing damage to cardiomyocytes 15. Besides the severity of elevation was in correlation with the extent of myocardial damage 19. To this context non-elevated cnTI levels observed in this study, altough in advanced stages of the infection, might be briefly explained with nonprogressed or undeveloped myocardial injury.
Silvestrini et al 17 detected that cTnI levels were higher (p=0.017) in proteinuric dogs compared with nonproteinuric dogs with CVL. Hypoalbuminemia and proteinuria, prominent features of CVL, classically exist secondary to glomerulonephritis due to immune complex deposition 20. Similar pathogenesis could affect microvasculature damage as in the myocardium, probably accompanying to cardiomyocyte damage and subsequent cnTI release 17. However in the present study taking into account UPC values in different stages of CVL, proteinuric dogs (stage II, III and IV) did not present cTnI elevations, not in agreement with Silvestrini et al 17. On the other hand only a limited portion of dogs participated in this study would have had influence on results.
As myocarditis and arrhythmias have been shown to occur in human beings and dogs with leishmaniasis 20, ECG and ECHO examination might be of beneficial for noninvasive detection of myocardial injury. Given ECG as a noninvasive detection method 21 and it usage both for diagnostic and prognostic purposes in suspected myocarditis cases 22, the present project established 12 lead computerized ECG for supporting the diagnosis and detection of myocardial injury and foremost cardiac alterations (cardiac monitorization, atrial/ventriculer dilatation, congestive heart failure, conduction disturbances, arrythmia, pericardial disease) in relation with CVL.
Electrocardiograms of 105 dogs serologically positive CVL infected dogs were examined for rhythm disturbances and alterations in ECG waves 20. Sinus arrest (14.3%), right bundle branch block (4.8%), and atrial premature beats (4.8%) were observed. Those latter alterations were suggestive of enlargements of left atrium/ventricle and myocardial hypoxia 20. Another study evaluated the cardiotoxic changes in relation with pentavalent antimonial compounds in CVL 23. Twenty-eight dogs naturally infected with Leishmania infantum were treated with meglumine antimoniate and ECG examination before and after treatment were determined, in which no abnormalities were detected on ECG tracings 23.
In the present study increased T wave amplitude (voltage) [more than 1/3 of the amplitude of the R wave, not composed of biphasic T-wave] and ST segment elevation [>0.15 mV] were evident in two (stage III) and three cases (stage IV). Both changes might be dedicated to myocardial infarction and myocardial hypoxia 23. It should be better to make interpretation along with ECHO examinations. Entire ECG evaluation revealed mild sinus tachycardia and atrial premature complex in two cases (stage II), mild sinus tachycardia and persistent sinus tachycardia each one case (stage III), mild sinus tachycardia, persistent sinus tachycardia and bradycardia each one case (stage IV), interestingly denoting that as stages of the disease develope ECG alterations rose.
ECG abnormalities by severity revealed that the vast majority with three cases were enrolled in stage IV (G IV) CVL infected dogs accompanied by moderate alterations. Twelve lead ECG abnormalities, which was not previously described in dogs with CVL, were detected in 6/28 (moderate or severe alterations) diseased dogs in this study. Additionally it was not easy to comparatively evaluate the results with previous studies performed six lead ECG.
Echocardiographic abnormalities, which was not previously described, both in M mode and B mode, in dogs with CVL were detected in more than two-third of L. infantum-infected dogs in this study. Left ventricular dilation and hypertrophy might be in relationship with myocardial injury 14. Left ventricular dilation is well known compensatory mechanism for preventation against decrease in cardiac output. Left ventricular dilation frequently preexist a persistent decline in FS 14, which was detected in six out of 28 cases (from stage I to III) of the L. infantum-infected dogs.
Systolic function decreases might be proofed via reduced fractional shortening (FS%) and ejection fraction (<45%). The latter two parameters are frequently analyzed for measuring the left ventricular systolic function, also suggested as a good indicator of ventricular contractility 24.
In an attempt to diagnose myocardial failure and specific chamber dilatation 2D/ M-mode echocardiography is required. Regarding veterinary literature, the vast majority of researches reported fractional shortening (FS) as foremost indicator of systolic function. Among dogs FS values less than 20-25% are suggested to be abnormally low, denoting systolic dysfunction. On the other hand even if mitral regurgitation is evident, FS may be misevaluated 25.
Studies regarding healthy dogs revealed FS (%)>%30 26 and values under should probably denote dilated cardiomyopthy 25. In the present study mean values among groups although did not reveal significant increase or decrease individual evaluation presented decreased FS in 1 case (29%) in GII, 2 cases in GIII (25% and 16.4%) and 3 in G IV (27%, 26.9% and 29.8%). As stages of CVL progressed independently from the serum biochemical findings, cases with decreased FS values elevated [3 out of 7 cases (42.85%) in stage IV]. However it was not able to attribute decreased FS values with dilated cardiyomyopathy. Besides in progress of dilated cardiomyopathy radiographical (not involved in the present study), ECHO and post-mortem (solely intravital diagnosis was evidenced, none of the cases were dead, hence this examination was impossible) examinations along with left ventricular and left atrial dilation might exist 26,27. Furthermore precise diagnosis of dilated cardiomyopathy requires entire evaluation of (a) left ventricular dilatation (b) decreased systolic function and (c) increased sphericity of the left ventricle 26,27. In our study among 28 dogs with CVL, six of those presented reduced FS values, might be attributable to systolic dysfunction.
Ejection fraction (EF) has been described as the foremost parameter for assessment of systolic function and values ranging from less than 40%-50% are denoted abnormal in humans. Considering dogs, an EF less than 40% is abnormally low 25 which indicates systolic function loss. In our study mean EF (%) values indicated no decrease, whereas individual examination revealed one case in stage III (% 35.3), which could be attributed to systolic function loss.
Left ventricular internal diameter at end diastole ans systole might be of beneficial for direct interpretation of cardiomyopathy, besides for indirect evaluation of cardiac chambers/congenital defects Regarding hypertrophic cardiomyopathy, LVIDd/ LVIDs both decreases, whereas in dilated cardiomyopathy both of those increase 28. In the present study both LVIDd and LVIDs values decreased in two cases of stage II, three in stage III and four in stage IV, respectively, which might be briefly explained with the occurence of hypertrophic cardiomyopathy. It may be briefly explained as the stage of CVL increased; progression of chronic disease might cause more severe cardiac injury, resulting in much more cases with decreased LVIDd and LVIDs values.
Tai ve Huang 29 in a prior study described enlarged left atria (1.5 ≤ LA/Ao ≤ 2), markedly enlarged left atria (LA/Ao > 2) based on left atrial/aortic root ratio (LA/Ao) (29). In our study enlarged left atria [one case in stage I (1.71), two cases in stage III (1.52, 1.61) and five cases (1.63, 1.77, 1.96, 1.75 ve 1.66) in stage IV] and markedly enlarged left atria only in one case of stage IV (2.06) were detected.
In conclusion based on Leishvet guidelines serological (IFAT titers and ELISA test kits), clinical and laboratory findings (especially Tp, Alb and UPC) cases were enrolled into four different stages/groups (stage I to IV) presented hematologic [altered erythrocyte markers], electrocardiographic [left atrial/ventricular dilation, myocardial hypoxia] and echocardiographic [left atrial enlargement, LVIDd increase/decrease, LVIDs increase/decrease, FS and EF decrease (systolic dysfunction)] alterations which must be taken into consideration. Cardiac status should be established which could contribute to intravital diagnosis and hasten additional therapy protocoles directed to cardiovascular system in dogs with CVL.