INTRODUCTION
The last decades of the past century, the most employed method in order to prevent diseases and improve the alimentary efficiency, was the use of antibiotics. Nevertheless, antibiotics may have negative influences because they could provoke resistance of pathogens to some drugs, such as, the presence of residues of medicines in animal made products. Also, affects the flora presented in the organism provoking a microbiological imbalance in the host 1.
Probiotics are live microorganisms (bacteria and yeast), and they are employed with the goal of improve the health and the productive performance in the host. For this reason, today is suggested to administer microorganisms with a probiotic effect in animal production as an alternative to reduce the diarrheic disorders, improve the animal health and increase the productivity 2). Probiotics microorganisms can be developed from simple cultures or mixing bacteria with yeast; and, when applied to animals or the man, they have a positive benefit in their health 3. Also, probiotics stimulate the immune system and they take part in the correction of the microbial natural balance of the host 2. Nevertheless, the obtaining mode of probiotics is not totally clear, so, evaluation is essential in order to optimize their use 4. According to previous studies, these microorganisms could adhere and survive in the gastrointestinal tract of the animals, where they act stabilizing and protecting the inner ecosystem 5.
Probiotics microorganisms can be found in different habitats such as soil, water, human and gastrointestinal tract, and in fermented products and food 4. The most commonly used genres in the development of probiotics prepares of veterinary use are: Lactobacillus, Bifidobacterium, Estreptococcus, Leuconostoc, Pediococcus, Propionibacterium and Bacillus, such as yeasts of the genres Saccharomyces, S. boulardii and Kluyveromyces3.
In this days several studies prove the goodness that agro-industrial residues have with previous treatment, to develop biological products of veterinary use, being suitable as additives in animal alimentation, such as a source of microbial development because they contain low concentrations of soluble sugars and a high protean value 3,5.
An important aspect in the development of probiotic products is the selection of an appropriated and financial faceable culture medium for productions. Different probiotic strains, generally, require different culture media according to their physiological characteristics 3. These media may be very complex and expensive, like the MRS medium 6 for lactic acid bacteria. According to Miranda 7 the agro-industrial sub products (molasses, milk serum, soy milk, vinasse) are available sources that can be employed with efficiency for the growing and development of the microorganisms with probiotic activities. The objective of the present study was to obtain, characterize and evaluate two bio-prepares developed from sugar cane molasses - orange vinasse fermented with yeast and or lactic acid bacteria.
MATERIALS AND METHODS
Site of study. Experimental work was performed at the Bromatology and Microbiology laboratories, Science Faculty, Escuela Superior Politécnica de Chimborazo, Animal production and industrialization laboratories, Engineering Faculty, Universidad Nacional de Chimborazo (Riobamba, Ecuador) and Microbiology Laboratory, Agricultural Sciences Faculty, Universidad Central “Marta Abreu” de Las Villas (Cuba).
Raw material characteristics. Orange vinasse is characterized for have a 20%(v/v) Dry matter (DM), 2.5% (v/v) Ash, 19%(v/v) Crude protein (CP), 10%(v/v) True protein (TP) and a pH of 3.56. Molasse presented an 85%(m/v) DM, 1.1% (m/m) Ash, 2.7% (m/v) CP, 0.8% (m/v) TP, pH of 5.8 and 72º Brix.
Selection, activation of strains and biomass obtaining. Selected strains for the obtaining of probiotic capacity prepares was: Kluyveromyces fragiis (L-4 UCLV) from the Microorganisms Bank of the Universidad Central “Marta Abreu” de Las Villas and four strains ATCC (American Type Cultures Collection, EEUU); Lactobacillus acidophillus 4356, Lactobacillus bulgaricus 11842, Streptococcus thermophilus 19258 and Saccharomyces cerevisiae 9763. The strains in freeze-dried format, were activated individually in an Erlenmeyer of 250 mL of capacity which contained 120 mL of triptona soy culture medium (BD Trypticase™, BDL™. 211768, EEUU) at 37°C for the case of bacteria and 30°C for yeast, in a stove with an orbital agitator (Inkubationshaube TH 15, Germany) at 60 rpm for 6 h. They were next growth in a dish with a culture medium Agar MRS (Man, Rogosa and Sharpe M6411-500G, HEMEDIA®, India) and Nutritive (Nutr, 213000-BD Disco™, EEUU) for L. acidophilus, L. bulgaricus and S. thermophilus, respectively. Agar Sabouraud was used for the yeast (Sabrd 211584-BD BBL™, EEUU). The lactobacilli were grown under anaerobic conditions using the container (GasPak Plus™).
Once the microorganisms were activated, the obtaining of two biomasses was next. T1, was composed by 0.05 mg (Radwag Analytic Scale AS 220/C/2, Switzerland) of biomass obtained from the affluent growing of which one of the bacteria strains, L. acidophilus (9.3x10⁷ UFC/mL), L. bulgaricus (8.9x10⁸ UFC/mL) and S. thermophilus (9.2x10⁸ UFC/mL), cultured in selective media (MRS and Nutr). T2, contained lactic bacteria from biomass T1 plus S. cerevisiae (9.3x10⁷ UFC/mL) and K. fragilies (L-4 UCLV) (9.4x10⁷ UFC/mL) cultured on Agar Sabrd. The mixes of this biomasses where inoculated in an Erlenmeyer with a capacity of 500 mL that contained 250 mL of sterile cow milk at 30±2°C and was incubated at 37°C for 24 h. The mixes of these micro-organisms were inoculated in an Erlenmeyer with a capacity of 500 mL that contained 250 mL of sterile cow milk at 30±2°C and was incubated at 37°C for 24 h. Finally, an initial recount was performed on the dish to verify the viability of the strains.
Obtaining of the probiotic prepared candidates. In an Erlenmeyer of 2.0 L of capacity, 600 g of sugar cane molasses and 1.00 L of orange vinasse was added, these where homogenized at 150 rpm with a magnetic agitator (JOAN o OEM, MS001, CN; Switzerland) at 28°C for 10 minutes, at the end of this time the molasses-vinasse substrate contained 78º Brix. Continuously 250g (9.2x10⁷ UFC/mL MRS, 9.3x10⁷ UFC/mL Nutr.) and (9.4x10⁸ UFC/mL MRS, 9.3x10⁷ UFC/mL Nutr. 9.5x10⁸ UFC/mL and Sabrd) of the biomasses T1 and T2 previously obtained separately were inoculated for the development of the T1 and T2 bio-prepares respectively. Five repetitions were performed for each bio-prepare. After incubation at 37°C for 24 h, in a stove (Memmert UN 30 PLUS, 1942794, Germany), in a 500 mL capacity Erlenmeyer 250 mL of the bioprepares was added (T1 and T2), in study and separately for its respective characterization.
Chemical composition, pH and instrumental color. CP and TP determination were performed following the methodology described by Dadvar et al 8; the DM, Ash and the ethereal extract (EE) was determined by AOAC methods 9. Simultaneously, in a parallel sample the evolution of pH since the biomass blending with the agroindustrial wastes was analyzed every hour until 24 hours of incubation at 37°C. In a pHmetro (HANNA® *H 110, USA) was used. The instrumental color was measured with a colorimeter (CR-400, Konika Minolta, Japan) in the CIELab* system with a wave length of 440 nm, 10 nm of resolution and through color comparison with HTML Code.
Microbiological analysis. From each bioprepares 5.0 mL were taken and both was homogenized with a physiological saline solution with a ratio of 1/10 (v/v). Serial dilutions were performed of (1/10, (v/v)) until 0.5 scale of the MacFarland scheme. The samples were planted in Petri dishes with Agar MRS, Nutr. y Sabrd. media. Then, were incubated for 24 h at 37°C and 30°C for bacteria and yeast, respectively. The number of colony former units (CFU/mL) was quantified by visual counting of the colonies.
Bile salts tolerance. In order to evaluate the bile salts tolerance, a culture medium was prepared, with nutritive culture medium supplemented with bile salts (Bile Oxgall Difco®, USA) in a concentration of 0.35% (w/v). The bioprepares were inoculated (T1 and T2) by separately in a concentration of 9.5x109 UFC/mL and were incubated for 24 h at 37°C. Subsequently, recounts were made in Petri dishes using the techniques described by Ortiz et al 10.
Gastric secretions resistance. Determine the resistance to the gastric secretions, this one was artificially prepared using the technique described by Ortiz et al 10. The control was adjusted to a pH of 6.5-7.0 with NaOH 5 N. The sterilization was made using membrane filtration of 22 µm. In the artificial gastric secretion and the control were inoculated with the probiotics with a concentration of 9.8x109 UFC/mL. Continuously was proceed to incubate at 37°C, and samples were toked 24 h after inoculation. Lastly, recounts were made through the technique used by Rodríguez-González 11.
Catalase activity. Measure of the catalase test was performed according Rodríguez-González 11, using hydrogen peroxide at 30% (v/v) over the grown colonies in the different culture media.
Hemolysis test. Measure of the catalase test was performed according Rodríguez-González 11, using hydrogen peroxide at 30% (v/v) over the grown colonies in the different culture media.
Origin, maintenance and activation of the pathogen microorganism’s indicators. As indicator pathogen microorganisms was used Escherichia coli spp. Salmonella spp. Streptococcus aureus. Obtained from the bank of strains of the Microbiology Laboratory, Natural and Exact Sciences Faculty, Pontifical Catholic University of Ecuador, Quito, Ecuador. The isolated were conserved at -80°C in a BHI culture medium with glycerol (Brain Heart Infusion Difco®, EEUU).
Antagonism determination of the bioprepared candidates to probiotics. Treatment T1 and T2, previously activated separately in a nutritive culture medium (234000-BD Difco™, EEUU), were inoculated in Petri dishes with Agar MRS, Nutr. and Sabrd, according the technique described by Ortiz et al 10. Subsequently, the dishes were incubated at 37°C for 48 h. After the formation of visible colonies, 10 mL of BBL culture medium (212207 - BD BBL™ Enterococcosel™, EEUU) were poured on the dishes, which contained 0.01 mL of the indicator pathogen strain with a concentration of 9.2x10⁷ UFC/mL and then were incubated at 37°C for 48 h. The antagonist activity was verified for the formation of transparent zones around the colonies greater than 1.0 mm. In order to verify the nature of the inhibition the described technique of Rizzello et al 13 was performed.
Susceptibility to antimicrobial agents. The modified method of diffusion in an impregnated disk (Bioanalyse®, USA) was used. The used antimicrobial was: Ampicillin (AMC) 10 µg, Gentamicin (CN) 10 µg, Streptomycin (S) 300 µg, Erythromycin (E) 15 µg and Penicillin G (P) 10 µg. An Escherichia coli spp. strain was used as control
A proportion of 0.1-0.2 mL of each bioprepares, corresponding to a cellular concentration of 9.8x109 UFC/mL, was distributed into the Petri dishes that contained Agar MRS, Nutr. and Sabrd for the bio-prepares T1 and T2. Meanwhile, for the control strain, the Agar BBL (211221-BD BBL™, EEUU) were used. With that purpose the technique of superficial spreading of the inoculate was employed and then the antibiotic dishes were placed. Were incubated for 48 h under anaerobic conditions for MRS and aerobic for the rest. After incubation, the inhibition halos were measured through the techniques described by Sourav and Arijit 14 and Rodríguez-González 11.
Statistical analysis. The statistical Software STATGRAPHICS Centurion 15.1 was employed for the statistical treatment of data through a variance analysis ANOVA according to a completely randomized design 15.
RESULTS
Physicochemical characteristics of the prepares candidates to probiotics. The half values of color, organoleptic and pH of the prepares candidates to probiotics are resumed on table 1. These presented a low luminosity color with a medium rate of yellow and red, with a chrome that was in the first quadrant, which results in a dark brown color. Both probiotics presented a bittersweet smell and taste, of liquid consistence and acid pH. There were no significant differences (p<0.05) between these bioprepares.
Table 1 Organoleptic characteristics and pH of the bio-prepares T1 and T2.
| Indicator | Treatments | ||||
|---|---|---|---|---|---|
| T1 | T2 | ||||
| Color (CIELab* System) | L 31.85 | a* 11.48 | L 31.93 | a* 11.42 | |
| C* 27.08 | b* 24.52 | C* 26.41 | b* 23.81 | ||
| H 64.91 | H 65.31 | ||||
| Color (código HTML) | #603883B | #613873B | |||
| Flavour | Bittersweet | Bittersweet | |||
| Smell | Bitter | Bitter | |||
| Texture | Liquid | Liquid | |||
| pH | 3.88 ± 0.12 | 3.85 ± 0.02 | |||
| Different letters in the same line differ (p<0.05); T1, L. acidophilus + L. bulgaricus y S. thermophilus. T2, L. acidophilus + L. bulgaricus + S. thermophilus + S. cerevisiae y K. fragilis (L-4 UCLV). L: Luminosity, a*: Red index, b*: Yellow index, C*: chrome H: Hue angle; HTML: color comparison. | |||||
Table 2 are shown the half values of the bromatological composition of the bio-prepares (T1 and T2). The DM and CP were greater (p<0.05) in T2. Meanwhile the As, EE and TP, did not alter (p>0.05) between treatments.
Table 2 Bromatological characteristics of the treatment T1 and T2.
| Indicator | Treatments | EE ± P | |
|---|---|---|---|
| T1 | T2 | ||
| Dry Matter (% HB) | 18.12b | 19.12a | 0.12 |
| Organic Matter (%DM) | 3.14 | 3.15 | 0.01 |
| Ethereal extract (%DM) | 3.22 | 3.25 | 0.25 |
| Crude Protein (%DM) | 17.23b | 18.51a | 0.34 |
| True Protein (%DM) | 10.80 | 12.20 | 0.31 |
| Different letters in the same line differ (p<0.05). T1, L. acidophilus + L. bulgaricus y S. thermophilus. T2, L. acidophilus + L. bulgaricus + S. thermophilus + S. cerevisiae y K. fragilis (L-4 UCLV). DM: Dry Matter. HB: Humidity base | |||
Figure 1, the kinetic of pH over the first 24 h of fermentation can be appreciated. The initial values of pH in both treatments were higher than 4, these were reduced below 4, independently (p>0.05) to treatment, in the first 24 h post incubation.
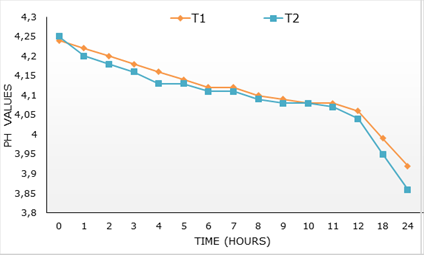
Figure 1 pH kinetic during 24 hours of both bio-prepares. T1, L. acidophilus + L. bulgaricus y S. thermophilus. T2, L. acidophilus + L. bulgaricus + S. thermophilus + S. cerevisiae y K. fragilis (L-4 UCLV).
Table 3, a microbial concentration is shown, the viability, and in vitro tests of the two biopreparet (T1 and T2), cultured in selective media (MRS, Nutr. and Sabrd) for their growing. At the moment of obtaining, the bioprepares presented a microbial concentration and a viability of 9.3x10⁸ UFC/mL and 93% respectively, in T1. In the in vitro tests, the microorganisms used to obtain two biopreparations showed resistance to gastric juice conditions at 0.35% and bile salts at 0.30%. In addition, they presented negative catalase and hemolytic ganma.
Table 3 Microbial concentration, viability, and in vitro tests of bio-prepares T1 and T2, in selective culture media.
| Indicators | Treatments | ||||
|---|---|---|---|---|---|
| T1 | T2 | ||||
| Culture media | Culture media | ||||
| MRS | Nutr | MRS | Nutr | Sabrd | |
| Microbial concentration (logX/mL) | 9.3x108 ±0.02b | 9.2x108 ±0.21b | 9.6x109 ±0.06a | 9.6x109 ±0.03a | 9.5x109 ±0.31a |
| Viability (%) | 92 ±0.12c | 92 ±0.02c | 94 ±0.21a | 93 ±0.11b | 94 ±0.01a |
| Lactic-Acid (mmol/mL) | 64 ±0.01b | 63 ±0.04b | 72 ±0.03a | 71 ±0.12a | 72 ±0.06a |
| Gastric Secretions 0.35% v/v | + | + | + | + | + |
| Bile Salts 0.30% | + | + | + | + | + |
| Catalase | - | - | - | - | |
| Hemolysis | ɤ | ɤ | ɤ | ɤ | ɤ |
| a,b Different letters in the same line differ (p<0.05); T1, L. acidophilus + L. bulgaricus y S. thermophilus. T2, L. acidophilus + L. bulgaricus + S. thermophilus + S. cerevisiae y K. fragilis (L-4 UCLV); MRS, agar milke sharpe. Nutr, Nutritive Agar. Sabrd, Sabouraud Agar; (+) and (-) possitive and/or resistent and negative. ɤ, gamma hemolytic | |||||
The lactic acid levels differ (p<0.05) between variants. Which explains the higher diminution of pH in the prepares candidate to probiotics.
Antagonist activity and antimicrobial susceptibility.Table 4 the antagonist activity of the two treatments against E. coli spp., Salmonella spp. and S. aureus, is presented, there were no difference (p<0.05) between T1 and T2.
Table 4 Antagonist activity (IH: inhibition halo, mm, IZD: inhibition zone diameter) of the bio-prepares T1 and T2, against strains of indicator pathogens.
| Indicator Strains | Treatments | |||
|---|---|---|---|---|
| T1 | IZD | T2 | IZD | |
| E coli spp | 23.6 ± 0.22b | ++ | 25.7 ± 0.12a | +++ |
| Salmonella spp | 20.2 ± 0.18b | ++ | 21.45 ± 0.08a | ++ |
| S aureus spp | 26.2 ± 0.12 | +++ | 26.5 ± 0.03 | +++ |
| a,b Different letters in the same line differ (p<0.05); T1, L. acidophilus + L. bulgaricus y S. thermophilus. T2, L. acidophilus + L. bulgaricus + S. thermophilus + S. cerevisiae y K. fragilis (L-4 UCLV); IH, inhibition halos; ++, 20-25 mm; +++, 25-30 mm of IZD. | ||||
Bioprepared (T1 and T2) employed in this study resulted sensitive to the used antibiotics. This shows that the bioprepares are no capable of generate antibiotic resistance to those drugs, as can be appreciated on table 5.
Table 5 Half values of halo diameter of anti-microbial susceptibility of the probiotic bioprepared (n=5).
| Antibióticos | T1 (HI mm) | T2 (HI mm) |
|---|---|---|
| Ampicilina | 10.2 ± 0.02b | 0.7 ± 0.02a |
| Gentamicina | 11.4 ± 0.03b | 10.5 ± 0.01a |
| Eritromicina | 12.2 ± 0.11b | 11.1 ± 0.12a |
| amoxicilina | 10.1 ± 0.23 | 0.9 ± 0.08 |
| Penicilina G | 0.8 ± 0.05a | 10.5 ± 0.03b |
| Estreptomicina | 11.5 ± 0.01 | 11.3 ± 0.12 |
| a,b Different letters in the same line differ (p<0.05). T1, L. acidophilus + L. bulgaricus and S. thermophilus. T2, L. acidophilus + L. bulgaricus + S. thermophilus + S. cerevisiae and K. fragilis (L-4 UCLV); IH: inhibition halos. mm, milimeter |
DISCUSION
Results obtained according to physical characteristics in both treatments in the present study (Table 1) are owed fundamentally to the employ of the agroindustrial waste, the brown color, in the different tonalities is owed to the sugar cane molasses, also the sweet flavor and the bitter scent are owed to the used substrate and to the formation of weak acids of small chain in the first 24 hours. Making a product accepted by animals 7,12. Similar bioprepares (BIOPRANAL) was reported by Marin 16. The inclusion of these bioprepares to the animal diet will improve the animal health, by stimulate the immune system and inhibiting the growing of pathogen agents 3,17. Some bacterial species and ideal yeasts for the development of probiotics were mentioned by Sánchez et al 18, which help to control the fermentation in agroindustrial waste 7,16,17.
The results of the chemical composition of the treatments (T1 and T2) obtained in the present study (Table 2), were owed to the substrates employed in the fermentation, the DM is due of the quantity of the employed micro-organisms dead cells (bacteria and yeasts), joined to water evaporation, at the end of the development cycle, in the same way the rising of CP and TP, is related with DM and the sedimented micro-organisms when finish the life time, meantime the values of EE are for the formation of weak acids of small chain and the ether formation during fermentation, nevertheless, the obtained levels in the present study are within the FAO permitted levels 5 for the microbial additives with probiotic activity. In a similar way, Sanchez et al 18 reported probiotics with CP and Ash contents equivalent to the obtained in the present study. Meanwhile, Miranda 7 indicated the existent relation between the substrate and the microorganisms employed in the nutritive content of the microbial prepares, in a way that, with the improvement of the substrate, the concentration of the fermented organic matter increases. By his part, Iyer et al 19 and Dadvar et al 8 reported that probiotic microorganisms are capable of form amino acids and peptides with biological activity, independently to the used raw material. While, Miranda 7 in previous studies, reported the effect of the physical factors and environments over the nutritive content of the microbial prepares.
One of the aspects acting on the reduction of pH in probiotics fermentations is the microbial specie, the employed substrates and the environment, in the obtention process 11,21. Meanwhile, Nazef et al 21 reported a pH inferior to 3.90 in microbial cultures, employing skim milk, Marin 16, obtained similar values using substrates derivate from sugar cane molasses, milk serum and torula yeast, agroindustry waste. By his part, Jurado et al 17 and Patil et al 22 improved the quality of the microbial prepared by reducing pH and the load of pathogen agents. But the pH values obtained in the present study are still inferior to the values reported by authors 4,7,11, when obtaining probiotics from pure or mixed strains
The microbiological results (Table 3), are owed to a homo fermentative developing of the employed micro-organisms, which provoked the improve of the lactic acid production in the microbial prepares obtained in the present study 8,25. The micro-organisms with the capacity to produce lactic acid in the fermented products help to control the growing of S. aureus and Clostridium26, fundamentally for the inhibitory action and the capacity of controlling the formation of gases in the nutritious bioproducts 19,23. By his part, Perez et al 25 reported the importance of know the quantity of micro-organisms introduced to the animal and the effect of these micro-organisms. Similar microbiological values were reported to the present study by Khalil et al 26 and Jacela et al 23 when employed sugar cane molasses and milk serum to ferment L. Acidophilllus and S. cerevisiae.
Mixed cultures developed in agroindustry waste and their antagonistic capacity to the pathogen microorganisms (Table 4), was owed to the action of the lactic-acid bacteria, through the production of weak acids of small chain, mainly the lactic acid, competing for the nutrients, their capacity to survive in anaerobic conditions, non-hostile environment for aerobic bacteria 27. At the same time, there are capable of produce lactic acid, hydrogen peroxide, possibly bacteriocins, which are characteristics of the metabolism of lactic-acid bacteria 13,24.
suggests that the bioprepares obtained in the present study could act against pathogen microorganisms 13,26. Similar result was reported by Marin 16 by employ L. acidophilus and K. fragilis, who observed changes in the proteins of the membranes that destroy the bile salts secreted by the enzymatic barrier, composed mainly of proteolytic enzymes such as pepsine, trypsin, and chemo-trypsin, those changes were lethal for multiple microorganisms such as E. coli. By his part, Heather et al 12 reported the resistance to bacteria and yeasts at different concentrations of gastric secretions. Meanwhile Rodriguez-Gonzalez 11 reported a microbial concentration of 6.5x10⁸ UFC/mL probiotic obtained from the Lactobacillus spp., sufficient amount to establish themselves in the gastrointestinal tract. Perez et al 25 observed in vitro tests the growing of Lactobacillus on gastric conditions. The bioprepares obtained through molasses-vinasse fermented with yeasts and/or lactic acid bacteria, were capable of inhibit the growing of E. coli, Salmonella spp. and S. aureus strains. Similar results were reported by Khalil et al 26 by employ mixed cultures of lactic bacteria and yeasts.
Negative catalase, of the bio-prepares is characteristic of probiotic organisms and agree with the reported by Le Blanc et al 20, they describes that lactic strains do not present catalase enzyme. Rodríguez-González 11 and Ortiz et al 10, recommends perform in vitro tests before apply to the animals in order to rule out the existence of side effects and/or negatives of bio-prepares with probiotic activity 17,22,24.
In the test of antimicrobial susceptibility between antibiotics and the two bio-prepares (table 5), was observed that in both cases there are sensibility to all the antibiotics that were used. The anterior allows to predict that the use of mixed cultures developed through lactic-acid bacteria and yeasts selected in this research, would be recommended to perform in vivo studies to evidence their use as an alternative for antibiotics of veterinary usage 8,13. The sensitivity of the strains could depend of the resistance to some antibiotic concentrations due to the presence of some plasmids, or to particular properties of the cell wall and membrane of these microorganisms, by a non-specific mechanism which act against different antibiotics and by the modifications in the encoder gene, making them impermeable 10,21,27.
Under the conditions of this study it can be concluded, the bioprepares obtained through sugar cane molasses - orange vinasse fermented with yeasts and/or lactic acid bacteria proved physiochemical, micro-biological properties with proper conditions for probiotic products. In in vitro tests a potential use as a probiotic was demonstrated by accomplish the basic parameters, such as: acid pH resistance, bile salts, broad spectrum of anti-microbial activity and inhibitory effect to the E. coli spp., Salmonella spp. and S. aureus.














