INTRODUCTION
Meat quality is a main concern for livestock industries and consumers’ needs. Some of the most important sensory attributes of meats are appearance, juiciness, flavour and tenderness 1. Previous studies have shown that the meat tenderization process is complex and could be affected by several different pathways including pre- and post-slaughter factors and their interaction 2.
The implementation of high-throughput analytical tools over the last two decades was an important step toward a better understanding of the complex biological systems that define muscle to meat conversion. It is well recognized that the biochemical postmorten processes are key steps in meat tenderization 3. At present, tenderization is unanimously regarded as an enzymatic process of proteolytic systems. Numerous authors suggest that calpains are the only proteases responsible for meat tenderization 4. Thus, numerous studies have focused on the factors influencing meat tenderness and their relation with calpain system. The present revision highlight the importance of the use of molecular approaches to unravel the mechanisms behind the variations in meat tenderization. The exploitation of these methodologies could deepen our understanding of the biological processes driving meat production. The thorough knowledge of the molecular mechanisms of the muscle-to-meat conversion would have a strong economic impact thanks to the improvements of the quality of the final products that would increase consumers’ purchase.
CALPAIN SYSTEM
The calpain system in skeletal muscle comprises two Ca2+ dependent proteases, calpain 1 and 2, and a third polypeptide, calpastatin, whose only known function is to inhibit the two calpains. This system has a number of different roles in cells, including but not limited to the “remodeling” of cytoskeletal attachments to the plasma membrane during cell fusion and cell motility, the proteolytic modification of molecules in signal transduction pathways, the degradation of enzymes controlling progression through the cell cycle, the regulation of gene expression, substrate degradation in some apoptotic pathways, and involvement in long-term potentiation 5.
Calpains. Calpains are Ca2+-dependent, cysteine proteases. So far, 15 calpains have been described in mammalian 6 The two most often described proteases are CAPN1 and CAPN2, termed µ- and m-calpain, alluding to their micromolar and millimolar Ca2+ requirement 5. Both proteases are heterodimeric, each composed of an 80 kDa catalytic subunit and a regulatory subunit of 28kDa 7.
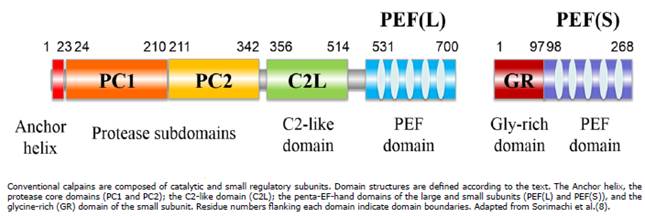
The 80 kDa subunits are different gene products (genes on chromosomes 29 and 16 in bovine, respectively). These subunits can be divided into four domains on the basis of their amino acid: the N-terminal anchor helix region; the protease core domains: PC1, an NH2-terminal domain, and PC2, the catalytic domain with a triad residue (Cys-His-Asn) characteristic of cysteine proteases; the C2-like domain (C2L); and the penta-EF-hand domain of the large subunit PEF(L) 8 (Figure 1). The 28 kDa subunit, common to both calpains, has two domains: the GR (glycine-rich) and PEF of the small (S) subunit 8 (Figure 1).
Calpains 1,2 have been detected in every vertebrate cell carefully examined for their presence 5. Different tissues/cells, however, differ widely in their protein ratios. Inmunolocalization studies have shown that both, CAPN1 and CAPN2, are located intracellularly along the Z disk/I band regions in the form of intracellular stores 9. Raynaud et al 10 found that CAPN1 is concentrated on the N1 and N2 line region of titin and suggested that this might constitute a reservoir for the cell. Many calpain substrates, including titin, nebulin, filamin, troponin-T and desmin, which attaches the sarcolemma to the Z-disc, are co-localized and proteolyzed during meat tenderization 9. Although calpains are considered to be cytoplasmic enzymes, recent researches have shown that they are also presents in several subcellular organelles such as caveolae vesicles 11, endoplasmic reticulum (ER) 12,13, mitochondria 14, Golgi apparatus and nucleus 15,16 showing the multifunction of this family.
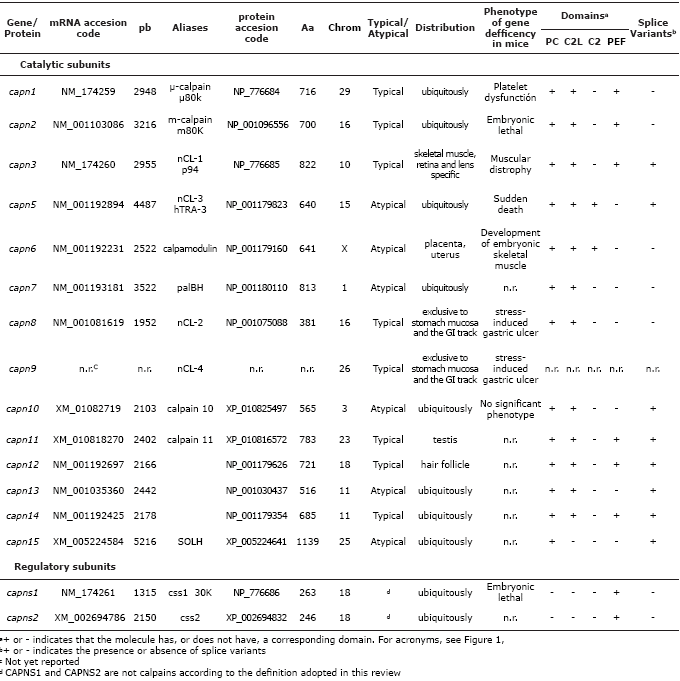
Due to the recent increase in the number of calpain-like molecules, Goll et al 5 proposed a nomenclature system. In this system, calpain genes have been named numerically, capn1 through capn15, and the polypeptides encoded by these genes have also be named numerically, CAPN1 through CAPN15 (Table 1). At present, two small subunits, CAPNS1 and CAPNS2, have been identified as well. The CAPNS1 subunit is an absolute requirement for the stability of both conventional calpain catalytic subunits in vivo, whereas CAPNS2, similar to CAPNS1, has no clear physiological clear role to date 8.
Calpains, based on their domain composition, can be divided into two general classes: “typical” calpains, those possessing a calmodulin-like domain at their C-terminus, and “atypical” calpains, those lacking a calmodulin-like domain IV at their COOH terminus. Considering their tissue distribution, calpains can also be classified into ubiquitous or tissue-specific calpains 7. The calpain-like molecules reported in bovine to date are described in Table 1.
Calpastatin. Calpastatin (CAST) is the only known endogenous protein inhibitor for calpains. Although only a single calpastatin gene exists in humans, pigs, mice and bovine, more than eight calpastatin isoforms have been identified in the tissues of those organisms, suggesting different levels of translation start at the promoter or alternative splicing mechanisms 6,17.
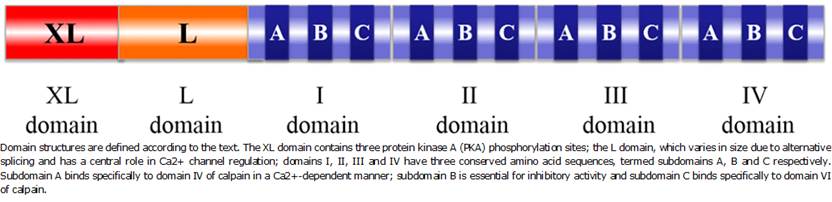
The genomic sequence of bovine CAST contains 35 exons spanning nearly 130 kp 18. Based on amino acid sequences, six different domains can be recognized. The XL domain, presents three protein kinase A (PKA) phosphorylation sites, the L domain, which varies in size due to alternative splicing and has been reported to be involved in binding calpastatin to biological membranes having a central role in the regulation of Ca2+ channel 19.
Domains I, II, III and IV can inhibit proteolytic activity of either CAPN1 or CAPN2. Each domain has three conserved amino acid sequences, termed subdomains A, B and C respectively. Subdomain A is a 14- amino acid sequence which binds specifically to domain IV of calpain in a Ca2+-dependent manner 7. Subdomain B is a 12-amino acid sequence essential for inhibitory activity. Subdomain C is also a 14-amino acid sequence that binds specifically to domain VI of calpain (Figure 2). The intact protein is capable of simultaneously binding to and inhibiting four calpain molecules 20.
The variation in meat tenderness between species, breeds and individuals is partly responsible for the variation in calpastatin expression and differential transcriptional activity of calpastatin gene promoters 21, therefore it is very important to determine its concentration.
MOLECULAR APPROACH
In this section we highlight data collected about the calpain system polymorphisms at DNA level, expression at RNA level and activity at protein level, and their involvement in the tenderization process.
DNA polymorphisms. There are many single nucleotide polymorphisms (SNPs), however in this review, we will focused in those that are used in commercial tenderness test.
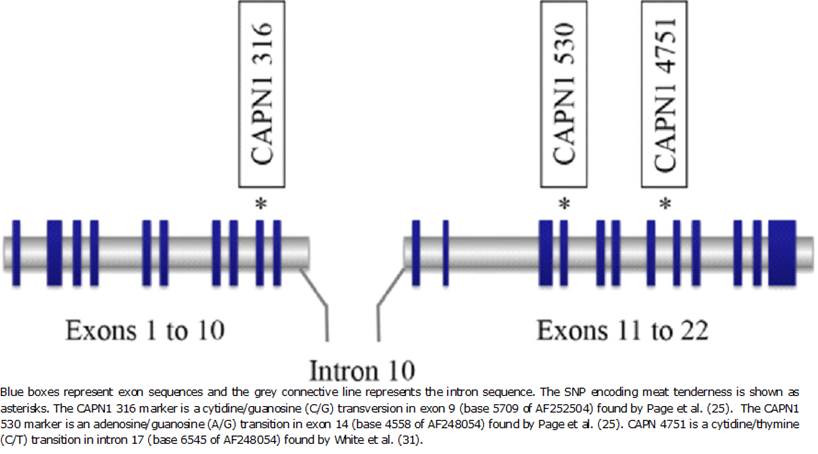
In 2000, the bovine capn1 gene was mapped to the telomeric end of BTA29 (Bos taurus autosome 29) 22 and a QTL (quantitative trait locus) for tenderness was found to segregate in this region 23,24. The markers CAPN1 316, a guanine to cytosine transversion in exon 14 (CC, CG or GG genotype) and CAPN1 530, an adenine to guanine transition in exon 9 (AA, AG, or GG genotype) were identified and associated with shear force values in Bos Taurus25(Figure 3). Animals homozygous for the C allele at marker 316 had lower shear force than animals of the CG or GG genotype while animals with homozygous G genotype at marker 530 had lower shear force than animals of the AG or AA genotype 26,27. The groups of animals, which were also analyzed with both markers fitted simultaneously, showed that specimens homozygous for the C/G haplotype are associated with the most favourable shear force phenotype. Nevertheless, these markers were not validated in Bos indicus animals 28,29. In Argentinean breeds, contrary to previous studies, steers inheriting the AG genotype at marker CAPN1 530 had lower shear force 30.
The new marker CAPN1 4751 was useful in populations of Bos taurus, Bos indicus, or Bos indicus x Bos taurus crossbred cattle 28,31,32 (Figure 3). Several studies in animals from different breeds demonstrated that those with the CC genotype at CAPN1 4751 had more tender meat than those inheriting the TT genotype 32-34. However, other authors found that the most favourable genotype in Bos indicus and its crosses with Bos taurus were CT alleles 35.
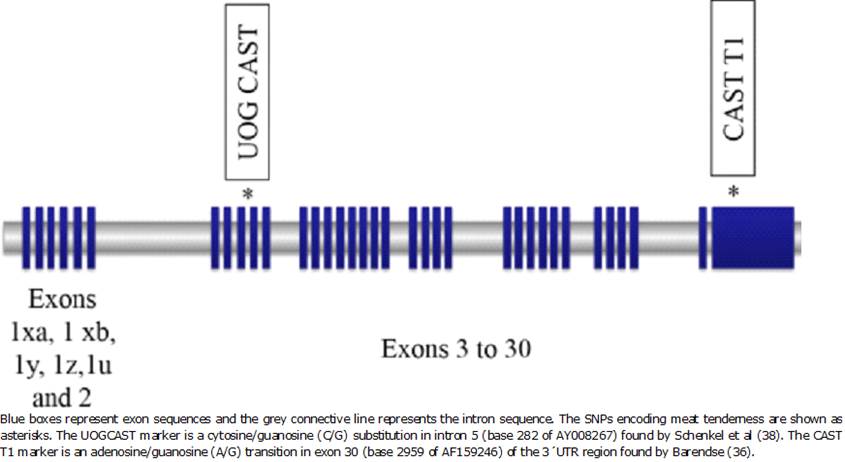
The cast gene, mapped to BTA7, is considered a candidate gene for beef tenderness. Several SNPs were found in calpastatin gene 29,36-38. One adenine to guanine transition in the exon 30/3´UTR region, termed as CAST T1; one guanine to cytosine transversion intron 5, termed UOGCAST, and a cytosine to thymine transition in exon 3 called WSUCAST (Figure 4). Schenkel et al. found a relationship between meat tenderness, measured as WBSF, and genotype in Bos taurus38. Genotype CC yielded beef that was more tender than GG while CG showed intermediate tenderness. All these results indicate that the effects of the markers are breed-specific and cannot be extended to all breeds 29.
Epistasis studies to evaluate possible interactions between SNPs on the CAPN1 gene and SNPs on the CAST gene were conducted and demonstrated that marker CAPN1 4751 has a significant additive-by-additive interaction with the markers WSUCAST and UOGCAST 32. These studies suggest that WSUCAST and UOGCAST are linked and are probably heredity like haplotypes.
RNA expression. The expressions of calpains and calpastatin at the mRNA and protein levels have been often determined in an attempt to understand the role of the calpain system in myofibrillar protein metabolism and meat tenderization. Generally decreased calpain expression or increased calpastatin expression is associated with toughness meat 39.
Some authors suggest that muscle type and/or fiber type can influence calpain and calpastatin expression and/or activities and therefore could account for post mortem proteolysis and meat tenderization 39-41. Differences in the small subunit (CAPNS1) and variants in CAST expression were found in slow-type muscles compared to fast-type muscles 40. Calpastatin gene expression has an average of nearly 30% in high WBSF groups compared with low WBSF at 6 days postmortem 42. Moreover, calpain-1 mRNA abundance was slightly higher and calpastatin mRNA was lower in longissimus lumborum than infraspinatus41, suggesting that the higher proportion of glycolytic fibers could improve the tenderness of certain muscles by accelerating post mortem aging due to the presence of a higher calpain/calpastatin ratio 43. Other researcher reveal that the slightly lower tenderness of meat from Zebu animals is probably not a result of lower expression of genes encoding proteases, but rather is due to the increased expression of CAST 44.
Some researchers found that gender alter mRNA levels of the calpain system. They show that bulls had lower WBSF values than heifers, which were accompanied by higher levels of CAPN1 and similar levels of CAST 45. Suggesting that variation in beef tenderness could be modulated through the differential expression of the members of the calpain system.
The maternal energy status during gestation alters meat tenderness. In a recent study, Jennings et al. found up-regulation of CAPN1 in longissimus muscle of high diet treatment foetuses 46. Feeding strategies have also impact in meat tenderness. Supplementation with vitamin D and zilpaterol hydrochloride had no significant impact on the expression of calpain-1 and calpastatin mRNA levels 47. Nevertheless, in previous studies in ours laboratories, the supplementation with corn silage during finishing alter calpains (1 and 2) and calpastatin expression, and meat tenderness, suggesting that managing feeding system has a regulatory effect on biological processes that occur in the muscle, defining the final quality of the meat.
The relationship between polymorphism described above and gene expression was also evaluated. Niciura et al 48 found that CAST was expressed twice as much in muscle of homozygous GG as in heterozygous AG in the CAST T1 marker. Natrass et al 49 investigated this relationship in Bos indicus and Bos taurus cattle and found differences in CAPN1 and CAST gene expression between favourable and unfavourable allelic variants of these genes (CAPN1 4751 and CAST T1 markers), indicating a polymorphic effect on gene expression. These findings suggest that differences in tenderness explained by gene markers could be a consequence of the alteration in their mRNA levels, protein activity and rate and (or) the extent of postmortem proteolysis in skeletal muscle. Studies should try to identify genetic and epigenetic events that may control the differential gene expression attributed to polymorphism.
Protein quantification and activity. Although Ca2+ -dependent proteases were identified in 1964, until the 90s there were no details of the procedures for extraction and determination of calpain proteolytic system activities.
It has been demonstrated that pH and temperature cause dramatic effects on CAPN1 autolysis and activity 50,51. Increased temperature or decreased pH cause a more rapid decline in CAPN1 activity. Calpastatin activity also decreased at elevated temperatures 51,52 while its inhibition of CAPN1 was apparently uninfluenced by pH 50. It is important to note that different temperatures and pH were studied by these authors, but during postmortem meat tenderness these parameters vary together; studies to perform these analyses are needed to determine the overall effect generated during aging.
During the storage period, the evidence suggest that CAPN1 and CAST activities declined significantly, whereas CAPN2 activity remained stable 9,53,54. In a recent research, calpain-1 activity was detected up to 42 days of post-mortem, and autolyzed calpain-1 up to 70 days post-mortem. The calpain-2 activity increase during the aging period, peaking at day 42 before decreasing sharply at day 70 55. Differences in activity could be ascribed to the type of muscle chosen or the cattle breed. Although some researchers suggested that loss of CAPN1 and CAST activities is due to the proteolytic degradation of these molecules, while others attributed it to extensive autolysis of CAPN1 and the breakdown of CAST possibly by calpains 39,56, it is uncertain whether the masking effect proposed by Kristensen et al. is the real cause of the decline in calpastatin activity 57. These authors demonstrated that the calpain system proteins are stable during the frozen storage period at -20°C or -80°C and that calpastatin activity is underestimated. These results suggest that calpain 1 contribute to early posmortem tenderization improvement and calpain 2 is responsible for additional tenderization during extended aging.
Calpastatin activity in muscle from older animals is more persistent postmortem than in muscle from growing animals 58. This difference may contribute to decreased protein degradation and increased toughness of beef from mature cattle, even after aging. These authors, also found that CAPN1 activity per unit of CAST activity in the muscle from growing animals is greater than in mature animals. Tissues with a greater CAPN1: total CAST ratio is expected to allow more postmortem protein degradation than tissues with a lesser CAPN1: total CAST ratio. Differences between young bulls and steers in CAPN1 and CAPN2 protein quantification were also detected 59. This effect could be the result of sex hormone action as previous studies have shown 60.
Feeding strategies during the growing and finishing phases have also impact in protein expression and meat tenderness 61. A recently study suggest that calpains, and most probably CAPN1, are more active in longissimus dorsi than in infraspinatus 63. In recently studies in our laboratories, finishing animals with corn-silage produced changes in CAPN1 and CAST activity (not published). Feeding strategies can modulate muscle protein turnover, muscle energy levels at slaughter and water holding capacity; and consequently, can modify meat tenderness, the pH/T decline curve and sensory characteristics of meats. Thus, one of the goals to successfully implement a compensatory feeding approach is to establish the length of the compensatory period which results in the highest muscle protein degradation potential at the time of slaughter.
In conclusion, Calpain system plays a central role in postmortem proteolysis and meat tenderization. Studies over the last decade indicate that CAPN1, CAPN2 and CAST plays an important role in postmortem degradation of myofibrillar proteins and tenderization of muscle during refrigerated storage. Polymorphisms in CAPN1 and CAST were evaluated as tools to predict meat tenderness, nevertheless, the results indicate that the effects of the markers are breed-specific. Muscle type, fiber type, gender, age and feeding strategies can influence calpain and calpastatin expression and/or activities. Muscle conversion involves several complex processes influenced by pre- and postmortem handling. New studies should try to identify the genetic and epigenetic events that may control the differential gene expression to explain these processes. New researches should focus on understanding how these polymorphisms/genes/proteins interact with the environment or with other genes, affecting economic traits.