Introduction
Pancreatic disorders are common in small animals; however, they can be difficult to diagnose due to the anatomic inaccessibility of the pancreas, nonspecific clinical signs, and inconsistent laboratory findings. Therefore, ultrasonography evaluation has been increasingly used and is currently the method of choice in pancreatic assessment 1.
Ultrasonography is an important tool in the evaluation of pancreatic disorders in small animals. It was the first procedure to enable direct visualization of the pancreas in humans and it was then applied to animals. Recently, other modalities such as computed tomography, magnetic resonance imaging, and scintigraphy have been used to evaluate pancreatic disorders in humans 2. Nevertheless, ultrasonography remains the cheapest and most accessible, and technological advances have provided ultrasound devices with better image quality for pancreatic evaluation in small animals 3.
Pancreatic evaluation is completely operator-dependent; therefore, good knowledge of the anatomy of the pancreas is essential 3, even though new techniques such as Doppler, elastography, and microbubble contrast agents are being used to improve the accuracy of organ evaluation.
Acoustic radiation force impulse (ARFI) is a safe non-invasive technique that measures tissue stiffness through quantitative and qualitative methods 4-5. ARFI elastography was first used in Veterinary Medicine in the differentiation of prostate and breast tumors 6 and it is currently being tested in other organs.
Doppler provides real-time information on the vascular architecture and the hemodynamic aspects of vessels in various organs 7 while contrast-enhanced ultrasonography (CEU) evaluates the perfusion of modified tissues and the vascular pattern of lesions 8. In Medicine, this technique has been used to identify pancreatic tumors based on its vascular pattern 9 and has been very effective in detecting necrotizing and acute pancreatitis 10-11-12; however, few reports are available in Veterinary Medicine.
The aim of this study was to describe and report the usefulness of the ultrasonographic techniques available for the evaluation of the canine pancreas.
B-mode Ultrasonography
Patients subjected to B-mode ultrasonography should fast for at least 8 hours prior to the procedure so there are no interferences from gastric contents. Trichotomy should be broad, extending to the 8th and 9th intercostal spaces for intercostal organ visualization. In most cases, the patient should be in supine position; however, right and left lateral recumbency can help scanning 3. Evaluation can be performed using linear or convex transducers, at a frequency of 7.5 to 15 MHz in small and 5 to 8 MHz in large dogs.
Pancreas ultrasonographic evaluation has some limiting factors, such as the patient’s body condition, which may hinder the identification of the organ due to increased mesenteric fat; localized pain in patients with pancreatic disorders; gastrointestinal contents, which can impair organ visualization; and other factors particular to the pancreas such as its small size, poorly defined borders, and echogenicity similar to the adjacent mesenteric fat 3, giving it poor resolution 13.
The pancreas is a thin and elongated organ located along the greater curvature of the stomach and descending portion of the duodenum. It can be divided into three parts: right lobe, body, and left lobe. It is a homogeneous organ, isoechoic to the mesenteric fat and the caudal lobe, and slightly hyperechoic to the liver (Figure 1). The right lobe is long and narrow, 1 to 3 cm wide and 1 cm thick. The left lobe is shorter and wider 13-2. The diameter of the pancreatic duct is 0.4 to 0.8 mm in the left lobe and 0.5 to 0.9 mm in the right lobe 14.
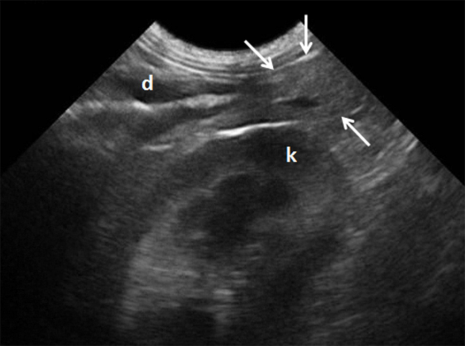
Figure 1 Ultrasonographic view of the right pancreatic lobe in a clinically healthy dog (arrows indicate organ). Pancreaticoduodenal vein viewed as an anechoic structure in the pancreas. (d) duodenum. (k) right kidney.
The pancreaticoduodenal vein can be seen in the right lobe and may be traced to the gastroduodenal and portal veins 13. Only the veins that drain the right lobe are visible by ultrasonographic evaluation 1. The right pancreatic lobe is located between the right kidney and the descending duodenum. Once the kidney has been identified (transducer positioned medially), the duodenum can be visualized adjacent to the abdominal wall. Alternatively, the pancreas can be detected by the pancreaticoduodenal vein, which can be found parallel to the descending duodenum 3-13.
The body of the pancreas extends caudally to the pyloric region, and the portal vein is located left and dorsally to it. The left lobe can be found between the stomach, spleen, and left kidney 13. It originates in the body and extends dorso-caudally to gastric antrum, continuing between the stomach and transverse colon. While in dogs the left lobe is hard to be visualized due to the gastrointestinal content, in cats it is more easily seen than the right lobe. In a study with 100 dogs, reported that the left lobe was only visualized in 57% of animals, as opposed to 87% for the right lobe. The large number of animals used in the study highlights the difficulties in visualizing the left pancreatic lobe in dogs 15.
The main disorders detected by ultrasound examination of the pancreas are pancreatitis, nodular hyperplasia, cysts, neoplasms, edema, and abscesses 3-13.
Pancreatitis is the most frequent disorder that affects the exocrine pancreas in dogs and requires safe and accurate techniques to obtain a quick prognosis and avoid complications to the patient. The complications most commonly observed are pancreatic cell death and regional blood supply loss, which can trigger systemic inflammatory response and lead to death 8. The ultrasonographic findings of pancreatitis vary with the severity, extent, and duration of inflammation in the tissue itself and in adjacent tissues. In acute pancreatitis, the organ is often enlarged, with reduced echogenicity, poor definition, and increased echogenicity of the adjacent mesentery (Figure 2). In some cases, free abdominal fluid may be present and the duodenum may be thickened and irregular. In dogs, the right pancreatic lobe is usually the most affected 13.
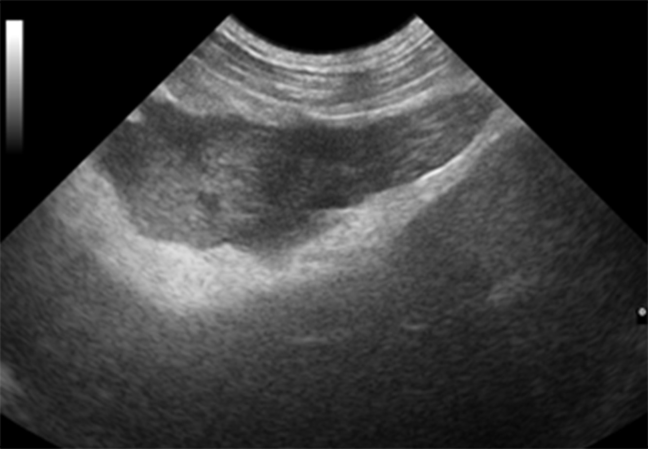
Figure 2 Acute pancreatitis in a dog. Enlarged pancreas with reduced echogenicity and increased echogenicity of the adjacent mesentery.
Acute pancreatitis does not always produce sufficient alterations to be visualized on ultrasound, especially in cats. Multifocal hypoechoic areas, cystic lesions, hyperechoic regions, and mixed echogenicity patterns can also be seen in pancreatitis; as well as edema, hemorrhage, and necrosis. In the sub-acute phase, cystic necrosis may develop, resulting in pseudocysts. Intramural edema and dilation or obstruction of the bile ducts have been reported in cases of acute pancreatitis 2. Chronic pancreatitis results from recurrent episodes of pancreatitis. The organ becomes hyperechoic and may be smaller. However, the larger the pancreas the greater the echogenicity and more irregular the contours, the more suggestive of chronic disease.
Nodular hyperplasia is commonly found in older dogs and is difficult to differentiate from neoplasia, the latter being typically greater than 2 cm and hyperplasia being smaller. In cases of pancreatic cystic lesions, differentiation of the various types of cystic pancreatitis requires histopathological analysis 2. Pancreatic masses are often poorly defined and vary in appearance according to their location (right lobe, body, or left lobe). Mass effect has been reported in cases of pancreatitis. In small animals, neoplasms are rare and difficult to differentiate from inflammatory processes. While in pancreatitis there is a diffuse reduction in pancreatic echogenicity, neoplasms are characterized by the presence of nodes or hypoechoic masses 3.
Doppler
The Doppler technology is a method that, when associated with conventional ultrasonography, provides real-time information on the vascular architecture and hemodynamic aspects of vessels. The hemodynamic parameters such as the systolic/diastolic ratio (S/D), resistance index (RI), peak systolic velocity (PSV), and pulsatility index (PI) enable comparison of flow during the phases of the cardiac cycle. These indexes are used to assist in the evaluation of stenosis, thrombosis, or in peripheral vessel analysis 7.
Previous studies using Doppler have reported good results and applicability in animals. In Veterinary Medicine, the effectiveness of this technique has been proven in hemodynamic studies of tissues and it has been fundamental in the assessment of neovascularization in tissue alterations 16. Furthermore, it can be used in early pregnancy diagnosis, gestational monitoring, and differentiation of benign and malignant breast tumors 16-18.
In contrast, few reports are available on the applicability of Doppler in the assessment of pancreatic vascular indexes in Veterinary Medicine and is mostly used to differentiate the pancreaticoduodenal vein from the pancreatic duct and to assess vascularization 13.
Colored Doppler is used to analyze the vascular characteristics of affected pancreatic parenchyma, including the presence or absence of neovascularization, vessel type (around, network, or mosaic), and their location (peri or intra-pancreatic).
In Medicine, studies have shown that Doppler ultrasound has increasingly contributed to the diagnosis and staging of pancreatic diseases, due to the significant increase in the sensitivity of this technique. Doppler is used to assess the intra and peri-pancreatic vasculature, such as the portal vein, splenic vessels, mesenteric vessels, and large vessels. Inflammatory masses are described as hypervascular; and carcinoma, the most common pancreatic neoplasm in humans, as hypovascular. Doppler has a sensitivity of 93% and specificity of 77% in cases of suspected masses and an accuracy of 88% pancreatic cancer diagnosis. In the presence of collateral circulation, these numbers increase to 97% sensitivity and 92% specificity, with an accuracy of 95% 19,20.
Contrast-enhanced Doppler ultrasonography appears to be useful in pancreatic assessment; however, further studies are needed. A study in cats has shown a significant increase in vascularity and blood volume in animals with pancreatic disease when compared to controls. Higher values were detected with power Doppler than with color Doppler, and were more effective at post-contrast than at pre-contrast time 21.
In Veterinary Medicine, few studies have been reported on the use of Doppler in pancreatic and peri-pancreatic assessment; however, further research is being done based on data from human medicine, in order to contribute to the evaluation and diagnosis of pancreatic disorders.
Acoustic Radiation Force Impulse (ARFI) Elastography. This ultrasound technique consists in the technological extension of one of the oldest medical tools, palpation, which has limitations in detecting deep masses relative to the skin surface, as well as other existing alterations in the diseased tissue, such as tissue density, water content, and acoustic interaction capacity. These limitations have led to the development of an area of diagnostic imaging that enables diagnosis beyond the limits of palpation 22. The results previously obtained using new ultrasound techniques have been promising, which has ensured their use in Veterinary Medicine, especially in dogs, due to their low cost and scientific similarity to humans.
Elastography was developed in the early nineties and is considered a promising method to assess the elasticity of tissues, enabling the study of tissue stiffness. Since then, various methods for assessing tissue elasticity have emerged (compression, acoustic radiation force impulse - ARFI, and real-time shear velocity - RSV), 4.
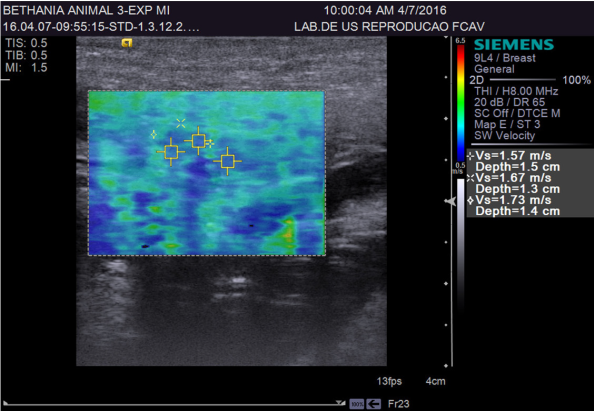
ARFI elastography provides quantitative and qualitative measurements of tissue stiffness with low interobserver variability 22. Quantitative ARFI employs a primary acoustic pulse towards the region of interest, promoting the formation of propagating pressure waves capable of deforming tissues, and the speed of propagation of these pressure waves (shear) is then measured. The propagation velocity and attenuation of waves are related to the stiffness and viscoelasticity of the tissue, with greater speed being observed in harder tissues 23(Figure 3).
Qualitative ARFI uses short high-intensity acoustic pulses that deform the tissue elements and create a static map (elastogram) of relative tissue stiffness. This diagnostic tool provides a grey scale map of the relative stiffness of the tissues from the studied area, which is then compared to the corresponding conventional ultrasound image. Generally, lighter areas represent more deformable tissues than dark areas 24.
In Medicine, elastography has been used mainly in the identification and differentiation of breast tumors, prostate tumor diagnosis, monitoring of focal and fibrotic hepatic lesions, the study of the structural properties of the kidneys 25, myocardial evaluation 26, musculoskeletal and gastrointestinal analysis 27, thyroid nodule research, characterization of atherosclerosis, and control of renal ablation results in human patients 28. In Veterinary Medicine, the use of ARFI elastography is new and experimental. It has been used in the evaluation of breast tumors in female dogs 5, standardization of reference values in the liver and kidney, and spleen evaluation in adult dogs 29.
A study using 52 healthy human patients and 46 with chronic pancreatitis evaluated by ARFI elastography reported that the pancreas of patients with chronic disease showed higher elasticity than those of healthy patients. Quantitative values obtained from these patients were 0.78 to 1.40 m/s. The positive, negative, sensitivity, and specificity predictive values obtained were 69, 78, 75, and 72%; respectively 30. In another study, the quantitative mean values reported were 1.28 m/s in normal pancreas, 1.25 m/s in chronic inflammatory disease, and 3.28 m/s in acute inflammatory disease 31.
Contrast Enhanced Ultrasound (CEUS). CEU is a recent imaging technique that uses stabilized gas bubbles to enhance the ultrasound image. Due to their high reflectiveness, the microbubbles agents are capable of increasing the Doppler signal, enabling the detection of flows hardly detectable by traditional methods 32.
Innovative studies in humans and in animals have shown that CEU is an imaging technique that can be used to assist B-mode and Doppler mapping in the hemodynamic evaluation of various organs, including the hemodynamic behavior of the canine pancreas. This technique has been used in Medicine for the assessment of myocardial viability, breast cancer detection in women, intestinal ischemia, peripheral arterial disease, hepatic vascular diseases, and renal perfusion studies 33; among others. In Veterinary Medicine, studies using this method are recent but its use has been reported in the evaluation of liver, spleen, kidney, prostate 34, and pancreatic tissues 8. In a study conducted in cats, testicles from healthy animals have also been evaluated 35.
This technique defines parameters related to homogeneous or heterogeneous filling of organs by microbubbles, mainly nodular areas within the abnormal tissue, enabling the classification of intensity patterns: high intensity (hyperenhanced), wherein the mass cannot be differentiated from the surrounding tissue (isoenhanced); and low intensity patterns (hypoenhanced) 36. It can also be used to evaluate vascular filling times, from the time of injection of the contrast in the bloodstream to the start of organ perfusion (wash-in); contrast peak (highlight), which is the highest moment of infusion; and total time of contrast output from the parenchyma (wash-out). This pattern of microvascular filling is of great importance in early detection of small masses at the early stages of evolution and hypervascularization of aggressive tumors, aiding non-invasively in the differentiation between malignant and benign neoplasms 37.
A recent study compared the perfusion characteristics and enhancement patterns of the pancreas in healthy dogs and those with pancreatitis using CEU. It was reported that in dogs with acute pancreatitis the mean quantity of pixels and peak intensity of the parenchyma were significantly higher than in normal dogs 8. Another recent study comparing contrast tomography with CEU in cases of acute pancreatitis concluded that ultrasonographic examination may be an alternative to contrast tomography and should be the method of choice 38. However, other alterations such as adenocarcinoma, fibrosis, pseudocysts, and pancreatic necrosis are difficult to be differentiated by this technique due to their lack of vascularization 8.
Furthermore, evaluated the use of CEU with microbubble agents in the pancreas of healthy cats, it was reported that the contrast allowed better definition of the pancreatic borders. This data may be useful as a reference for future studies in pancreatic disorders in cats 12.
Although the CEU is valuable in hemodynamic investigation of tissue perfusion, in the diagnosis of neoplastic diseases, and in traumatic or necrotic lesions due to differences of blood flow, histopathological analysis will always be the gold standard for the diagnosis of lesions 8.
Conclusion
Ultrasonography is an affordable and non-invasive technique that provides important information on organ parenchyma and adjacent structures. It is an essential tool in the diagnosis of pancreatic disorders and provides quick prognosis. In addition, new techniques are being increasingly studied to complement the information obtained by conventional ultrasound and contribute to better diagnostic quality.