INTRODUCTION
Mammary tumors are extremely common in clinical routine of small animals, being the type of tumor most frequently observed in dogs 1. Due to its elevated frequency, many studies have been performed attempting to understand the carcinogenic process, as well as the biological characteristics associated to mammary tumor, i.e., genetic, morphological and biochemistry alterations associated to it 2-5. In this sense, many molecular markers evaluated by immunohistochemistry, have been widely used as prognostic markers, and thus, determine the biological behavior of different types of cancer.
In veterinary medicine, markers such as Ki-67 (cell proliferation), E-cadherin (adhesion molecule), cyclooxygenase-2 (COX-2), estrogen (ER), progesterone (PR), HER-2, p-53 are targets of several researchers in the evaluation of canine mammary tumors 2,3,4. Ki-67 is a protein expressed in different cells during the replication phase, and since tumor cells have a high replication rate, Ki-67 is considered an excellent marker for diagnostic and prognostic of mammary tumors 5. E-cadherin is an adhesion molecule expressed by epithelial cells, such as the epithelial cells of the mammary tubes 6. The expression of this protein has been evaluated in several type of tumors, and a relationship between its decreased expression and aggressive neoplasia, with major metastatic potential and worse prognosis was demonstrated by many authors 6,7. COX-2 is an enzyme expressed during inflammatory stimuli, being very important for activation of the inflammation cascade. Also, the presence of this protein has been associated with tumor progression 8, and recently studies indicated that COX-2 was associated with higher metastatic potential and poor prognosis of breast cancer 6,8,9.
The etiology of breast cancer is considered multifactorial, i.e., hormonal, environmental, genetic and oxidative factors may be associated with the carcinogenesis processes 10. The exact mechanism that oxidative stress induces carcinogenesis is not well established; however, free radicals may contribute to lipid peroxidation and biochemical alterations, in addition to cell damage 11. Oxidative and free radicals formed by cellular metabolic activity, that could be associated with the carcinogenic process, are minimized by antioxidant mechanisms, which can be measured by ferric reducing ability of plasma (FRAP). It is important to empathize that individuals with cancer show reduced antioxidant levels, which contributes to disease progression 12.
Acetylcholinesterase (AChE) is an enzyme present in cholinergic and non-cholinergic tissues, being responsible for acetylcholine (ACh) hydrolysis in the post-synaptic cleft 13. ACh has an important role mediating inflammation, being able to attenuate the release of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), and interleukins (IL-1 and IL-6) 13,14. According to Das 14, the AChE activity can be considered a systemic marker of inflammation in several diseases, including cancer. Also, the AChE has been implicated in the tumorigenesis, since AChE can be amplified, mutated and/or highly expressed in many types of tumors, altering the AChE activity 15,16. Thus, the aim of this study was to evaluate the AChE activity and FRAP levels in dogs with mammary cancer before and after surgical procedure to remove these tumors, as well as the relation between these variables with immunohistochemical markers of tumor.
MATERIALS AND METHODS
Experimental animals and sampling. This study used 13 dogs with mammary tumors that were routinely attended by veterinarians from the clinical service and surgery of the Instituto Federal Catarinense. Total blood (10 mL) was collected in tubes with and without EDTA before surgery and 10 days after. Blood samples containing EDTA were aliquoted, diluted, and used for AChE assessment, haemogram, and platelet counts. Blood samples without EDTA were centrifuged at 3000 x g for 15 min to obtain serum to be used to evaluate biochemical variables (creatinine, alkaline phosphatase, alanine aminotransferase (ALT)), and FRAP levels.
The dogs were divided into two groups (A and B). The group A was formed by dogs with tumors smaller than 3 cm of diameter, and the group B was formed by dogs with tumors of 3 cm of diameter or larger. Tumor fragments were collected for histopathological and immunohistochemistry evaluation. Also, total blood was collected from seven healthy dogs (the control group) for AChE activity and FRAP levels assessment. Before surgical procedure, abdominal ultrasound and X-ray were performed to verify comorbidities and metastasis.
AChE activity. For AChE activity assessment, an aliquot of 40 µL of blood collected with EDTA was diluted in sodium phosphate buffer containing Triton X-100, according the methodology described by Worek and colleagues 17, where the AChE activity was measured at 436 nm in 37ºC. The method is based on the hydrolysis of acetylcholine substrate forming acetate thiocoline, which in turn reacts with 5.5’-dithiobis-(2-nitrobenzoic acid) (DTNB) to form a yellow anion 5-thio-2-nitrobenzoico. The color intensity is directly proportional to the quantity of product formed. The results were expressed in mU/µmol of Hg.
FRAP levels. FRAP levels in serum samples was performed according to the methodology described by Benzie and Strain (18), using a semi-automatized method (Cobas Mira Plus) as detailed described below. FRAP reagent (300 mL) freshly prepared was warmed to 37ºC and a reagent for blank reading was taken (M1) at 593 nm; 10 mL of sample was added, along a with 30 µL of H2O; at a final sample dilution of 1/34. Absorbance (A) readings were taken after 0.5 s and every 15 s thereafter, during the monitoring period. The change in absorbance (∆A593 nm) between the final reading selected and the M1 reading was calculated for each sample and related to ∆A593 nm of a FeII standard solution tested in parallel. The results were expresses in µmol/L.
Immunohistochemistry. For immunohistochemistry evaluation, the slides were immersed in methanol: hydrogen peroxide solution during 15 minutes to block endogenous peroxidases of the tissues, followed by two washes during 5 min in PBS solution. After, it was performed the antigen recuperation in humid heat at 90ºC (Steam Cuisine 700 Hi Spped-T-Fal?) for 20 min, followed by cooling at room temperature during 20 min. Blocking of unspecific reactions was performed with milk powder Molico? 5% diluted in PBS solution during 25 min. The slides were incubated overnight with primary antibody diluted with PBS solution. Primary antibodies for COX-2 - clone SP21 diluted 1:100 (Cell Marque?), Ε-cadherin - clone EP700Y diluted 1:300 (Cell Marque?) and Ki-67 - clone SP6 diluted 1:500 (Cell Marque?) were used. Goat anti-mouse Biotinylated diluted in PBS solution was used as a secondary antibody. This antibody was incubated during 30 min and performed washes during 5 minutes with PBS solution. Amplification and accentuation of reaction was performed using avidin-biotin peroxidase system (according manufacture recommendations - Horseradish Peroxidase - Dako?) for 30 min followed by the addition of diaminobenzidine (according to manufacturer´s recommendations - DAB Reagent Set KPL?) for 5 min, followed by water wash. Slides were stained by Harris hematoxylin (1:2) in distilled water during 1 min. Following, the slides were washed in water, dehydrated and mounted with coverslip in Entelan?. For the COX-2 marker were used normal intestinal mucosa as a positive control. For the Ki-67 marker were used normal lymph node as a positive control. For the E-cadherin marker were used normal epidermis as a positive control. Negative controls were assessed using NaCl 0.9% solution.
Histopathology. Three fragments were collected for histopathology. The samples were fixed in buffered formalin 10% solution, routinely processed, and sections stained with hematoxylin and eosin technique (HE). Neoplasic lesions were described according to Cassali and colleagues 1.
Statistical analysis. The date of seric parameters, tumors, and haemogram were analyzed using a descriptive statistical method; measures of central tendency (median) and dispersion (amplitude - difference of minimum and maximum value) were computed. For AChE activity and FRAP levels, the control group and pre and post-surgery groups were compared by Kruskal-Wallis H followed by Nemenyi post hoc test. Also, the parameters were related as variability of pre-surgical and post-surgical in consideration of groups A and B, and later groups A and B in relation of surgery effects using Mann-Whitney U. The difference between groups A and B on tumor size were performed by Mann-Whitney U. Haemogram and biochemical parameters (erythrocytes, hemoglobin, total leukocytes, platelets, creatinine, alkaline phosphatase, and alanine aminotransferase) were tested as differences between groups A and B (tumor size) using Mann-Whitney U. It was considered statistically different when p<0.05. All analyses were performed in Software R-Language v.2.15.2. (R Development Core Team, 2012).
In addition, it was tested the correlation between E-cadherin, Ki-67 and COX-2 and the AChE and FRAP by Point-Biserial correlation, an especial type of correlation that allows the use of dichotomy and continues variables. For this analysis, dichotomy variables were E-cadherin (code as 1 if it was tested negative and coded 2 if it was positive), Ki-67(coded as 1 if < 25% and coded as 2 if ≥ 25%) and COX-2 (coded as 1 if + and/or ++ and coded as 2 if > +++). In addition, Ki-67 levels were analysed as percentage (its raw form) and correlated (Pearson’s correlation) with the levels of E-cadherin, and COX-2. It was also correlated E-cadherin versus COX-2.
Ethics committee. All procedures were performed under the appropriate guidelines and with the approval of Ethics Committee for Animal Experimentation (CEUA/Instítuto Federal Catarinense (IFC), protocol 13/2015).
RESULTS
Clinical evaluation and tumor staging. The dogs showed no laboratory abnormalities, except thrombocytosis (not significant), such as demonstrated in Table 1. However, it was detected lung metastasis in one of the dogs (Group B) studied in the first evaluation.
Table 1 Results from haemogram, platelet counts, and biochemical variables (creatinine, alkaline phosphatase and alanine aminotransferase - ALT) from dogs with mammary cancer before and after surgical removal of tumor(s).
| Parameters | Group A | Group B | P | Reference |
|---|---|---|---|---|
| Erythrocytes (µL³) | 6.75 ±0.86 | 6.56 ±0.47 | 0.64 | 5.8-8.5 |
| Hemoglobin (g/dL) | 15.95 ±1.01 | 15.30 ±1.63 | 0.51 | 14.0-19.1 |
| Total leukocytes (mm³) | 9700 ±3658 | 12600 ±5632 | 0.44 | 4900-17000 |
| Platelets (mm³) | 306500 ±25630 | 358000 ±84200 | 0.39 | 181000-525000 |
| Creatinine (mg/dL) | 0.80 ±0.06 | 0.61 ±0.26 | 0.26 | 0.6-2.0 |
| Phosphatase (UI/L) | 54.70 ±12.6 | 58.0 ±6.9 | 0.87 | 12-121 |
| ALT (UI/L) | 55 ±18.5 | 40 ±15.7 | 0.85 | 18-86 |
| Results are presented as median ± standard deviation. There was no difference between groups considering p<0.05. |
Histopathological examination, degree of malignancy and immunohistochemistry. Out of 13 dogs, three showed benign and 10 showed malignant tumors, among which five were carcinoma, three mixed sarcomas, two papillary carcinomas, one tubular carcinoma and one anaplastic carcinoma. These results can be visualized in Table 2, which also includes the results of immunohistochemistry (Ki-67, E-cadherin, and COX-2) (Figure 1).
Table 2 Histological and immunohistochemical results of canine mammary tumors separated into two groups according to tumor size: the group A (tumors smaller than 3 cm) and the group B (tumors larger than 3 cm).
| Groups | Histological results | Ki-67 (%) | Cox-2 # | E-Cadherin # |
|---|---|---|---|---|
| A | Papillary carcinoma grade II | 74.00 | ++++ | ++++ |
| A | Being mixed tumor | 8.00 | ++ | +++++ |
| A | Papillary carcinoma grade III | 0.78 | + | +++++ |
| A | Being mixed tumor | NP* | NP* | - |
| A | Being mixed tumor | 6.00 | - | +++++ |
| B | Carcinoma in a mixed tumor grade III | 33.33 | +++++ | ++++ |
| B | Carcinoma in a mixed tumor grade I | 19.33 | + | ++++ |
| B | Carcinoma in a mixed tumor grade I | 34.00 | +++++ | +++++ |
| B | Sarcoma in a mixed tumor | 47.33 | ++ | - |
| B | Carcinoma in a mixed tumor grade I | 24.66 | +++++ | ++++ |
| B | Carcinoma in a mixed tumor grade I | 17.33 | ++++ | ++++ |
| B | Tubular carcinoma grade I | 32.00 | ++ | +++ |
| B | Anaplastic carcinoma | 54.00 | +++++ | - |
| B | Sarcoma in a mixed tumor | 27.33 | ||
| B | Sarcoma in a mixed tumor | 28.66 | ++++ | - |
| *NP - not performed; # concerning staining intensity: - (absent), + (weak), ++/+++ (intermediate), ++++/+++++ (strong). |
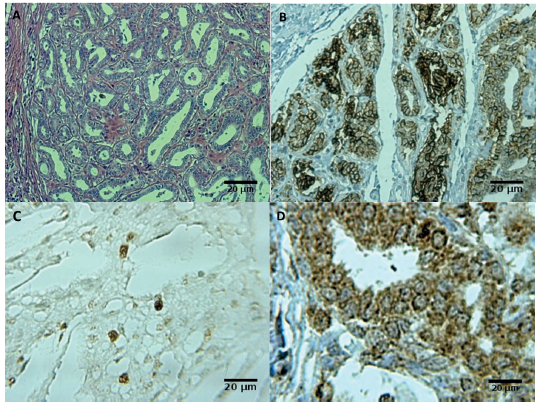
Figure. 1 Mammary tumor in dogs Canis familiares: Photomicrographs show sample of papillary carcinoma grade II characterized by papillary arborescent epithelial proliferation. Hematoxylin and eosin (A). The same papillary carcinoma showed a strong expression of E-cadherin (B), expression of the Ki-67 (C) and a strong expression of COX-2 (D).
AChE activity in total blood. The AChE activity was significantly higher (p<0.05) in dogs with mammary cancer compared to control animals (Figure 2A), but no differences (p>0.05) were observed before and after surgery (Figure 2B). Tumor size did not influence AChE activity, i.e., there was no difference (p>0.05) between groups A and B (Figure 2C).
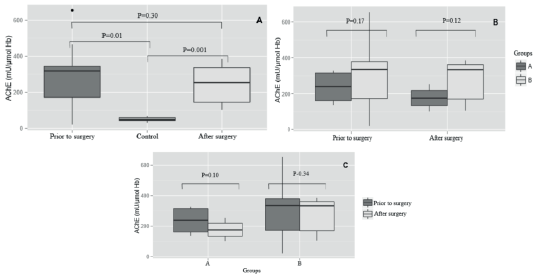
Figure 2 Acetylcholinesterase (AChE) activity in dogs with mammary tumor divided into two groups based on tumor size (A - tumor of at least 3 cm; B - tumor largerthan 3 cm): (a) AChE activity in total blood for dogs with mammary cancer (before and after surgery) compared to healthy dogs, regardless of tumor size. (b) Comparison within the group considering the before and after surgical removal.
FRAP levels in serum. FRAP levels before surgery were significantly lower (p<0.05) compared to control animals (Figure 3A). There was no differences on FRAP levels (p>0.05) between groups before and after tumor removal (p>0.05; Figure 3B). FRAP levels increased significantly after surgery in animals of the group A, a fact not observed in dogs from the group B (Figure 3C).
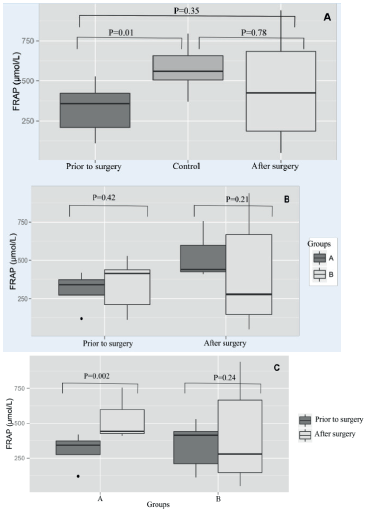
Figure 3 The ferric reducing ability plasmatic (FRAP) in dogs with mammary tumor divided into two groups based on tumor size (A - tumor of at least 3 cm; B - tumor larger than 3 cm): (a) Seric levels of FRAP from dogs with breast tumors (before and after surgery) compared to healthy dogs, regardless of tumor size. (b) Comparison between groups, according to the FRAP levels before and after surgery. (c) Comparison within the same group considering before and after surgical removal.
Correlation analyses. E-cadherin showed low, but significant positive correlation with FRAP levels (r=0.37, p=0.05), which means that samples positives for E-cadherin showed increased levels of FRAP. No correlation between E-cadherin and AChE was observed (p=0.21). The other marker (Ki-67) was correlated neither to FRAP nor to AChE (p=43, p=12), and finally the marker COX-2 showed a moderate significant positive correlation to FRAP (r=0.55, p<0.05), which means that higher levels of COX-2 (>+++) were able to increase FRAP levels. In addition, COX-2 showed a low significant positive correlation to AChE (r=0.32, p=0.01) (Table 3). Among markers, the only significant positive correlation was between Ki-67 and COX-2 (rho=0.51, p=0.04).
Table 3 Point-biserial correlation between acetylcholinesterase (AChE) activity and ferric reducing ability plasmatic (FRAP) levels with markers of diagnostic and prognostic of tumors (E-cadherin, Ki-67 and COX-2).
| Characteristic | Median AChE (min-max) | Median FRAP (min-max) |
|---|---|---|
| Total | 309 (0-655) | 357 (0-1582) |
| Correlation* with E-cadherin | -0.01 | 0.37* |
| Correlation* with Ki-67 | -0.06 | 0.01 |
| Correlation* with COX-2 | 0.32* | 0.55* |
| *Statistical significant correlation |
DISCUSSION
The results obtained in this study show, for the first time, a positive correlation between seric AChE activity molecular markers of dog with mammary tumors. Several studies have shown a relation between tumorigenesis and AChE activity, as well observed in this present work. The AChE exerts important functions in the central nervous system, but also is distributed in many tissues, and exerts other functions such as the capacity to regulate proliferation, differentiation, and apoptosis 13-15. The relation between cell cycle and AChE activity raises a number of questions about the role of AChE in tumorigenesis, since several studies have linked the cholinesterase to many tumor types. Also, increased AChE activity is observed in different tumor types 19,20. In this sense, the results observed in this present study are in accordance to results observed in the literature, where higher AChE activity was observed in dogs with mammary tumors compared to the control group. The variability of AChE activity in the group B can be explained by histopathological analyses that found different types of tumors. According to Martínez-Moreno and coworkers 19, the AChE activity may vary according to the histological type of tumor, explaining the wide variation on the AChE activity.
The AChE activity is also involved in the inflammation process 13,14. It is known that AChE has an anti-inflammatory role, and increased AChE activity could be associated with increased inflammation 21, such as observed in this present study due to the positive correlation between COX-2 levels and AChE activity. COX-2 is a protein expressed during inflammation, being associated to tumor development, and promotion of angiogenesis 22, associated to bad prognosis in dogs with mammary tumors 23. Also, we can observe that even with different histological tumor type, higher levels of the marker COX-2 were detected mainly in dogs with larger tumors. It has been demonstrated that tumor size has an important impact on prognosis and dogs survival 1,8. Although the range of survival of these animals was not established (study not finalized), it is believed that these dogs have a significant reduction on survival, impacted by the size of tumors and the strong marker COX-2, which leads to believe that this is an aggressive neoplasia 23,24. Ki-67 marker is used to evaluate cell proliferation, being associated with a worse prognosis 1,4. Thus, we believe that these two variables are associated, both indicating a bad prognosis.
Regarding E-cadherin, it is possible to state that many animals have a high labeling index, independently of the type or tumor size, unlike observed in other studies that a reduction in E-cadherin expression was observed in mostly aggressive neoplasias, being defined as an unfavorable prognostic marker 1,7. In the other hand, Ferreira et al 25 demonstrated that E-cadherin was expressed in both benign and malign cells, as well observed in this work. These same authors also demonstrated that reduction in E-cadherin levels shows a relation to high Ki-67 levels, and this could be associated with a bad prognosis. Nevertheless, there is no data that suggests a direct functional interaction between these markers, and so, the association between them and an unfavorable prognosis is uncertain. Brunetti and collaborates 2 demonstrated that reduction in expression of E-cadherin would be associated with a greater invasiveness of the tumor, but not associated with major tumor proliferation, presenting a small influence on animal survival. In this study, it was not observed correlation between these markers, what may have happened in consequence of reduced number of animals in this experimental design. Apparently, an inverse relation between Ki-67 and E-cadherin was not observed, however, in dogs with anaplastic carcinoma, considered an aggressive tumor, there was no E-cadherin marker but Ki-67.
Oxidative stress has been implicated in the carcinogenesis on some types of tumors, and the levels of antioxidants can be associated to lower risk of disease 26. Although this study did not evaluate the parameters of oxidative stress, the antioxidant levels were produced to fight free radicals formed during the oxidation processes 27. Our results show that FRAP levels were lower in dogs with mammary tumor, however FRAP levels increased after treatment of animals from the group A. Similar results were observed by Evans and others 28, which demonstrated that cancer has the capacity of increasing oxidation and, consequently, decrease the antioxidant mechanism. On the other hand, Singh and coworkers 29 showed reduced FRAP levels after surgery and chemotherapy in woman with mammary tumors. According to these authors, the use of chemotherapic drugs, that leads to mitochondrial damage and increased reactive oxygen species (ROS), are responsible for reducing FRAP levels, as well observed by Sharma and colleagues 12. According to these authors, reduced FRAP levels may be associated with minor defense capacity of cells against cancer, possibly due to genetic alterations associated with tumorigenesis, also affecting the genes involved in protein synthesis associated with the antioxidant mechanisms. Also, the inflammatory process involved during tumorigenesis can affect the antioxidant system. Moreover, highest COX-2 expression and AChE activities were observed in patients with tumors, demonstrating the potential of cancer to be inflammatory stimuli.
In conclusion based on the results, we have verified a positive correlation between AChE activity and COX-2, and these could be associated to increased tumor inflammation, influencing cancer prognosis. Also, FRAP levels were significantly lower in dog with mammary tumors, contributing to increased oxidative stress. A strong presence of the marker COX-2 is observed in the most aggressive tumors, being positively correlated to Ki-67, which can be associated to a bad prognosis of these patients. In relation to E-cadherin, a higher variability in results was observed, not being possible to correlate this marker to others.














