INTRODUCTION
Testicles are male reproductive organs responsible for the primary sexual characteristics whose function is the production of sperm and sex hormones, especially testosterone. The feline testes are normally located within the scrotum and each testicle is round to oval in shape 1.
Disorders of the testicles are common in domestic cats, the changes including neoplastic processes, torsion, injures, atrophies and infection processes, such as orchitis and epididymitis 2 and the evaluation of the testes for morphologic abnormalities can assist in the diagnostic evaluation of infertility 3.
Testicular ultrasonography in cats enables the determination of the size, volume, position and internal constitution of the testicles 4. It is using to assess palpable and no palpable changes, to differentiate testicular from epididymal and scrotal disease, and to localize undescended testicles. However, histologic diagnoses cannot be made based on the ultrasonography appearance 5. The use of ultrasound is importance in testicles evaluation, owing the ability to detect anatomical modification and assist in procedures of ultrasound-guided such as aspiration and biopsy 6. Additionally, the knowledge and development of this technique in domestic cats can be very useful for applications in wild cats.
Doppler, ARFI (acoustic radiation force impulse) elastography and contrast-enhanced ultrasonography are non-invasive using novel ultrasonography-based imaging modalities can also contribute to testicles evaluation in animals 7-8. B-mode and Doppler ultrasound provide real time information on the vascular architecture and hemodynamic aspects of the vessels 3. Elastography is a promising technique that evaluates tissue elasticity, which provides quantitative and qualitative measurements of tissue stiffness, with reduced inter-observer variability 8. Microbubble contrast-enhanced uses contrast media of encapsulated inert structures, highly reflected by the apparatus that improve the color and spectral Doppler signal 9.
Given the importance of this ultrasound technique for reproduction of tom and results news with the application of others methods this review aims to describe the main applicability of ultrasonographic tools in testes of domestic cats.
B-mode ultrasonography. Ultrasonographic study of the testes is a common diagnostic imaging procedure. It has performed after clipping of the scrotum region 10. Some examiners recommend use of a standoff pad and ultrasound gel is preferred as a contact medium over alcohol because of the risk of scrotal irritation 11.
The testicles should be examined with a high frequency transducer (at least 7.5MHz) 5-11. A 5MHz or lower transducer may not provide sufficient resolution to detect small lesions or subtle parenchymal changes 5. In addition, a linear transducer with broad contact area and good resolution in the near field is preferable over a sector or curvilinear transducer. The testicles should be scanned in transverse, longitudinal, and dorsal planes 1,5,11.
The B-mode ultrasonography in testicular animals allows the determination of biometric values such as volume and size, assessment of topographic and parenchymal features, position and its internal constitution 4. In cats, the testes are readily located within the scrotum and they have easily located by ultrasound 1.
Normal testicles are of medium echogenicity and have a fine, homogeneous echotexture 12. The testicular border is characterized by a thin, smooth and hyperechoic tunica albuginea (Figure 1). On transverse images, the mediastinum testis appears as a centrally located hyperechoic focus and on sagittal images, a central hyperechoic line is visible that represents the mediastinum testis 11.
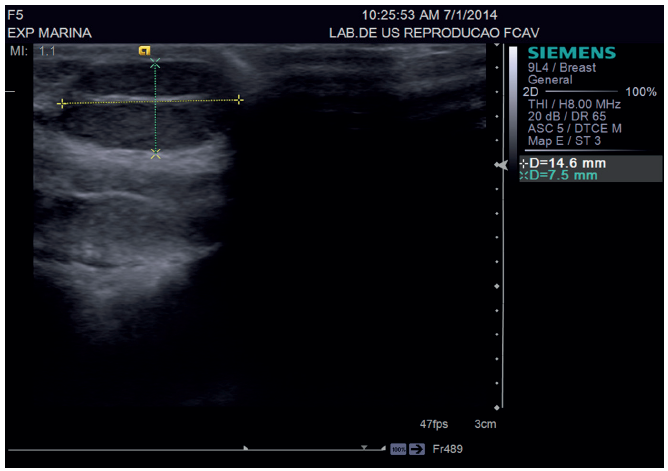
Figure 1 Normal testes in domestic cat, it must be measured during evaluation (callipers). The testes are an echogenic linear structure in the central portion of the testicle. The mediastinum testis appears as acentrally located hyperechoic focus and on sagittal images.
The epididymis is composed of a head, body and tail. The tail of the epididymis is generally less echoic than testicular parenchyma and has a coarser echo texture than testes. The head is cranially located, and from it the body can be followed caudally 5.
The testes should be imaged ultrasonographically whenever there is clinical evidence of urogenital tract disease or reproductive disorders. Testicular disease may be the source of the clinical signs, or concurrent disease processes may be present. The disorders include developmental disorders (cryptorchidism), inflammatory disorders (orchitis and epididymitis), testicular or epididymal cysts, testicular neoplasia, duct ectasia of the epididymis, infarction, atrophy, trauma and torsion. Other disease processes that affect the scrotum include hydrocele or hematocele and scrotal hernia 5,11,13.
It is therefore, relatively difficult, if not impossible to confidently differentiate benign vs. malignant nodules based on ultrasonographic appearance alone and tissue sampling is usually required to obtain a diagnosis 14. However, in most cases, ultrasonography is unable to differentiate the various canine testicular disorders and thus it must be used in conjunction with the animal’s reproductive history, complimentary laboratory tests and invasive diagnostic techniques such as fine needle aspiration cytology, testicular biopsy and histopathology 15.
Doppler ultrasonography. Doppler ultrasound provide real time information on the vascular architecture and hemodynamic aspects of the vessels 3. Doppler ultrasound method allows to study the anatomical features of the vessels and their functional characteristics related to vessel blood flow and functional data for blood flow (presence or absence, direction and flow velocity) 16.
Testicular Doppler is a very important method for the study of vascular features, providing findings of the vascular and hemodynamic aspects of testicular artery architecture 3 of the reproductive organs of humans and animals, especially in helping detecting the conditions that may cause fertility problems 17. It is sensitive indicators of compromised testicular blood flow is an example of testicular torsion 5.
In medicine, testicular Doppler is routinely used to determine the blood flow of the testicular artery, the diagnosis of testicular diseases and the study of spermatogenesis 18. In men, conventional gray-scale ultrasonography and color Doppler have evolved as the most important imaging techniques for scrotal anomalies 19 and are considered essential in the diagnosis of testicular tumor by guidelines 20. According to our reviewing, there are few studies available about testicles evaluation in veterinary science, such as in stallion 21, dogs 22,23 and only one report in cats 7.
Using the color Doppler, the detection of feline testicular artery are consistent, identified dorsally, between testicular, epididymal structures, and tortuous pattern. However, the identification of marginal and intratesticular portions of the testicular artery in cats are limited, probably due to the small testicular volume 7.
The main characteristics evaluated by Doppler mode are calculate vascular indices: peak systolic velocity (SV), end-diastolic velocity (DV) and resistance index (RI = [PSV - DV] / SV). These also present the advantage, compared to measurement of flow velocity, of being independent of insonation angle 3,24.
In cats, the spectral Doppler, the testicular artery showed characteristics waves of low resistivity, with low pulsatility and resistance, featuring flows with large and continuous systolic peaks and high-speed flow during diastole, typical of organs with continuous demand for blood. Regarding the vascular indices of the testicular artery in cats, the values for systolic and diastolic velocities and resistance index are (Left testicle SV: 6.73±2.78 and DV: 2.80 ± 1.50 cm/s and RI: 0.54 ± 0.12; Right testicle SV: 6.23 ± 2.34 and DV: 2.77 ± 1.36 and RI: 0.53 ± 0.12). As this feature depends on other factors such as vessel diameter and blood flow region, with no influence of the tissue evaluated size 7 (Figure 2).
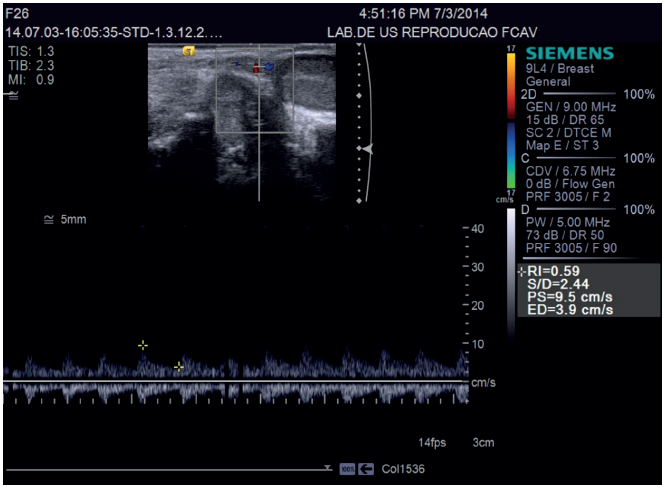
Figure 2 Color and spectral Doppler ultrasound image of the testicular in a tom. It is notice Doppler assessments to determinate of vascular indices in testicular artery
Contrast-enhanced ultrasonography. The microbubble ultrasound contrast is a new technique in veterinary science. It uses contrast media of encapsulated inert structures, highly reflected by the apparatus that improve the color and spectral Doppler signal 9.
Ultrasonography contrast agents consist of microbubbles, which are gas spheres of up to 10 mm in diameter, coated with a shell of different proteins, lipids, or polymers 25. Microbubbles are comparable in size and mechanical properties with the erythrocyte and cross-capillary beds but do not pass into the interstitial space, making them true intravascular agents 26.
The information obtained by contrast examination enables parameters related to homogeneous or heterogeneous tissue filling to be established 27. Furthermore, in conjunction with microbubble contrast-enhanced ultrasonography, B-mode the time for incoming phases (wash-in), output (wash-out) and peak enhancement of contrast-enhanced ultrasonography in tissues 28. The microbubble ultrasound contrast, in humans, in Europe and Asia and is established in cardiac and hepatic diseases, with applications in other organ systems, such as the testis, gaining wider recognition 29. This technique has been used in testicular diseases to obtain a higher degree of diagnostic accuracy when ultrasound findings are inconclusive 16. In case of tumors, lesions which have different perfusion of healthy tissue areas are highlighted differently by Contrast-enhanced ultrasonography 30; as well as the masses testicular has particular importance due to limited source of information that can be obtained as the invasiveness of tumors in this region, since tests involving sonographic techniques in B or Doppler mode has little sensitivity and specificity when compared to contrast technique using microbubbles 31.
In veterinary medicine, are few studies has been used to evaluate pancreas and lymph nodes 31, spleen 32-33, liver 34-35, kidneys 36, prostate 37. In testes, the first study on the use of the technique was performed in dogs 27. However, contrast-enhanced ultrasonography of the testicles of cats revealed mean wash-in time in healthy cats to be 8.78s and peak enhancement time 21.62s and wash-out time 75.36s. Contrast-enhanced ultrasonography of the left testicles revealed mean wash-in time 10.76s and peak enhancement time 21.50s and wash-out time 81.81s and of the right testis revealed (Figure 3). Furthermore, contrast fill the subcapsular vascular structures and after a few seconds, a homogeneous moderate enhancement of the parenchyma, with parenchymal vessels still distinguishable and after the peak phase, a rapid homogeneous decrease in echogenicity 7.
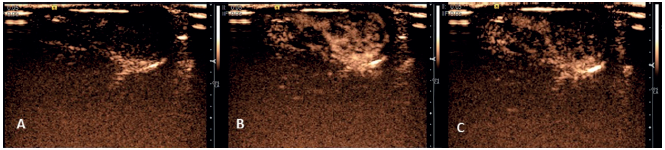
Figure 3 Ultrasound image of the testes of a healthy cat using contrast-enhanced ultrasonography. (A) wash in phase; (B) peak enhancement phase the homogeneous testes parenchyma can be visualized; (C) the wash - out phase.
Acoustic Radiation Force Impulse (ARFI) Elastography. Elastography is a new ultrasound technique that evaluates tissue elasticity. This ultrasound method consists of the technicization of one of the oldest medical practice tools, palpation, and thus evaluates the shape and stiffness of the organ of interest 38.Various methods for assessing tissue elasticity have been proposed, such as compression, acoustic radiation force impulse (ARFI) and real-time shear velocity (RSV) 39.
Acoustic radiation force impulse (ARFI) elastography is an ultrasonographic technique that provides quantitative and qualitative measurements of tissue stiffness, with reduced inter-observer variability 8 and this technique involves the generation of shear waves using radiation force impulse or shear force associated with a B-mode image 3.
In qualitative ARFI, short acoustic pulses and high intensities are used to deform the elements of the tissue and create a static map (elastogram) of the relative stiffness of the tissue. Alternatively, the quantitative approach to ARFI utilizes a primary acoustic impulse sent towards a region of interest and promoting the formation of pressure waves capable of deforming the tissues to raise the speed of the wave propagation (shear velocity). The wave velocity and the attenuation of acoustic pressure waves are both related to the rigidity and viscoelasticity of the tissue; the waves have a greater velocity in rigid tissues 40.
In humans, elastography has 100% sensitivity in detecting testicular tumors when values indicate an increased stiffness of the testicular stroma and facilitates the diagnosis of hematomas, cystic alterations, necrosis, calcification and other alterations that disrupt the homogeneity of the testicular tissue and can detect malignant lesions 41-42.
In veterinary medicine, the ARFI technique is a recent development and has been used to evaluate normal prostate and testes in dogs 8 testicular disorders in dogs 43 and testes in cats 44.
Qualitative elastography revealed that affected testicles had alterations in tissue stiffness and homogeneity. The values obtained for quantitative elastography of the testicular tissues were lower in degenerated testicles and hypoplastic, and the values were higher in testis with atrophied orchitis; leydigoma; sertolioma and interstitial cell tumours in dogs 43.
This technique was used to evaluate the testes of healthy cats providing novel information on testes elastographic parameters in clinically healthy cats, such as the quantitative values for tissue echogenicity, qualitative and quantitative findings of ARFI elastography. The testes examined by qualitative elastography were free of any malformations and the images appeared as homogeneous dark areas (Figure 4). The mean shear velocity values reported were 1.51 m/s (95% confidence interval: 1.42 and 1.59 m/s) for the right testicle and 1.48 m/s (95% confidence interval: 1.41 and 1.54 m/s) for the left testicle of the felines. There was no significant difference when comparing the right and left testicular structures 44 (Figure 5).
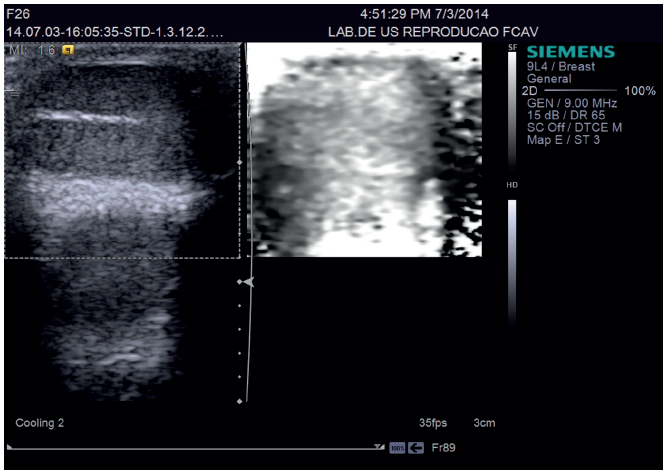
Figure 4 Ultrasonographic image of the testes in a healthy cat during qualitative Acoustic Radiation Force Impulse (ARFI) evaluation. (A) Testicular B-mode image. Qualitative ARFI elastography (arrows) of the testicle of a feline showing a homogeneous image (ligth-grey) that is typical and not pliable.
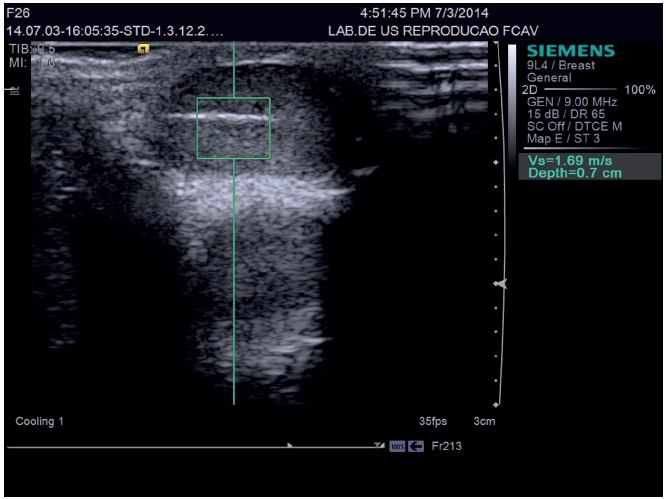
Figure 5 Image of quantitative ARFI elastography in the testis of a feline; Note the presence of the calliper within the testicular stroma for measuring the shear velocity.
Quantitative and qualitative ARFI elastography of the testes in cats is easily performed. Furthermore, the reference values for testicular elastography in healthy cats, the differences in shear velocity values of diseased tissues can be evaluated to differentiate between benign and malignant tumours in felines, once the definitive diagnosis of benign or malignant lesions is made only by histopathology after castration, considered an invasive method, which promotes the loss of the reproductive value of animals 44.
In conclusion, this review provides novel information on ultrasonographic methods used for testes exam in domestic cats. Testicular ultrasonography B- mode is useful to providing information on testicular parenchyma and allows to evaluate the determination of biometric while Doppler is emerging as an important and suitable non-invasive tool for blood flow assessment. Contrast-enhanced ultrasonography can also contribute to evaluation diagnostic tool for evaluating testicular abnormalities in sick cats. ARFI elastography of the testes in cats may provide valuable criteria for the evaluation and diagnosis of testes abnormalities and the differences in shear velocity values of diseased tissues can be evaluated to differentiate between benign and malignant tumors in felines. Once all of these new imaging methods are acknowledged in domestic cats, it can easily be applied to wildcat.














