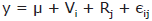INTRODUCTION
In Mexico, egg poultry farming is one of the most dynamic livestock sectors [1]. By adopting new technologies, productivity rates have risen, rising to the needs of the national market. Egg production in Mexico is comparable to that of developed countries; Mexico is responsible for 4.7% of the world's egg production, after China (45.2%), the United States (9.5%), India (8.2%), Russia (5.0%), and Japan (4.9%). Poultry farming is an economic activity of great national importance; in 2013, it accounted for 0.75% of Mexico's GDP, as well as 19.7% of the agricultural GDP and 42% of the livestock GDP. In addition, poultry farming is an important source of employment; at the end of 2013, it was reported that nearly 1.2 million jobs were directly and indirectly generated by Mexico's poultry farming industry. Eggs play an important part in the Mexican diet due to its low cost, as well as its high nutritional value and culinary versatility. Mexico has the highest per capita egg consumption in the world; in 2013, Mexicans consumed a reported 21.7 kg eggs/person [1].
Prebiotics are food additives that can be selectively fermented in the intestines leading to changes in the composition and/or activity of the intestinal microbiota with the goal of increasing health benefits [2]. Prebiotics can limit the growth of certain pathogenic organisms by supporting the colonization and growth of bifidobacteria, which provide health benefits to the host organism [2,3].
Oligofructans (linked simple sugars) are produced by many types of plants. They are concentrated or stored in the fine tissue of the plants, with the highest concentrations generally localized to roots and rhizomes. Among the oligofructans is inulin, a natural feed ingredient obtained from chicory root. Inulin is also present in other vegetables such as garlic, onions, artichoke, wheat, and agave. Inulin offers technological and nutritional benefits over other oligofructan additives and can be easily incorporated into a large range of products [4]. Inulin is obtained by means of an extraction process and is composed of a mixtures of oligosaccharides and polysaccharides; the degree of polymerization varies from 2 to 65 monomer units with a mean value of 10 units. Fructooligosaccharides (FOS) are the products obtained by enzyme hydrolysis of inulin, which have a degree of polymerization from 2 to 7 units with a mean value of 4 monomers [5].
The effectiveness of inulin or FOS in increasing poultry productivity depends on various factors. Variables such as the concentration of the prebiotic, the type of ration, characteristics of the animals and, above all, hygienic conditions and environmental stress, can influence the response of birds to inulin-type fructan feed additives [6].
Numerous studies in both animals and humans over the last twenty years have uncovered that inulin and FOS can stimulate the absorption of certain minerals. For example, the stimulation of Ca absorption has been documented in laboratory animals, in tests that included FOS [7-8], as well as in those that utilized inulin [9].
Polyamines are implicated in numerous biological reactions and are essential for cellular growth and proliferation. Polyamines participate in signal transduction and in distinct steps in the synthesis of AND, ARN, and proteins [8]. There are three polyamine sources: in vivo biosynthesis starting from amino acids, direct ingestion in the diet, and synthesis and/or liberation by the resident flora in the gastrointestinal tract [10].
Polyamine synthesis in eukaryotic cells starts with the decarboxylation of the amino acid ornithine to produce putrescine by the enzyme ornithine decarboxylase (ODC). An increase in the activity of ODC signifies an increase in the levels of polyamines, which can be used as a bio-indicator of stress. The modern demands for higher quantities of high quality, low cost eggs are pushing the egg production industry to seek better quality and food safety in their products. The objective of this work was to evaluate the effect of oligofructose from agave (OFA) supplementation to the feed of laying hens on hen development, egg laying, egg quality, and fecal polyamine levels.
MATERIALS AND METHODS
Experimental design. Eighteen weeks old Hy-line W-36 hens (n=300) were distributed at random in 3 treatment groups, further divided into four identical replicate groups of 25 hens each. The treatments were categorized based on their feed; the control group feed lacked OFA, and the OFA 0.1% and OFA 0.2% group feeds contained 0.1% and 0.2% OFA, respectively. The quantity of OFA required to obtain these levels was determined previously in previous experiments [8].
Feed and handling. The birds were housed in nesting boxes made of galvanized sheet metal containing corn cob bedding. During the study, the birds were given feed in the morning and in the afternoon in accordance with the nutritional requirements reported by the National Research Council [11]. The feed was served in hopper feeders, and water was provided in bell drinkers. For the OFA 0.1% and 0.2% groups, OFA was added to the feed in powder form. Total feed consumption was determined on a weekly basis and expressed as an average. Inoculations were carried out in accordance with the study zone and the technical reference of the avian strain. The period of light was adjusted to 17 hours.
Production and quality of eggs. Eggs were collected daily to determine the percentage of egg laying for each experimental group starting from week 10 of production. The evaluation of the quality of the egg was determined with a sample of 30 eggs per treatment at the beginning, peak and end of the posture. Egg quality was evaluated for the first 30 eggs, 30 eggs at the high point of egg laying, and the last 30 eggs for each hen. Haugh units [12] were calculated from egg weight and albumin height; albumin height was determined with a micrometer QCH with an 11 mm stainless steel calibration block. Eggs weight was determined on a digital balance. Shell quality was determined on an Egg Force scanner [13].
Egg quality was also assessed by determining the cholesterol content of the eggs. Ten eggs were chosen randomly during the initial egg laying period and at the high point of egg laying. For each test egg, cholesterol levels were measured in triplicate via high resolution liquid chromatography (HPLC) with UV and refractive index detection (HPLC-UV-RI) using a chromatographic method described previously [14]. This method uses an Agilent Technologies 1200 HPLC equipped with an SPD-10AV UV detector and an RID-10A refractive index detector. The 300 mm x 3.9 mm x 4 uní CN HP column was heated to 32°C [15]. Cholesterol levels were determined by comparison to external standards from 2.0 to 2.5 mg/mL cholesterol. The identity of the cholesterol peaks was confirmed by liquid chromatography with tandem mass spectrometry [16].
Polyamine levels. At the end of the study, ten fecal matter samples were collected from each hen to determine the presence of excreted polyamines by means of HPLC with fluorescence detection [17].
Statistical analysis. The experimental design consisted of a completely random model in which the treatment groups varied by the addition of OFA at different concentrations. The following model was used:
where:
y = the variable to be measured
μ = the general media
Vi = the i th level of addition of oligofructose
Rj = the j th effect of repetition
Є = standard error
The data were submitted to an analysis of variance test and the Fisher test of minimum significant variance with a 95% confidence interval for the variables of feed consumption, egg weight, percentage of egg laying, shell quality, Haugh units, and cholesterol levels. The statistical tests were performed using statistical software Minitab 16 Copyright 2014® [18].
Ethical aspects. The experimental protocol was revised and approved by the Research Coordination Office of the Academic Secretary, Universitary Centre of Bioilogical and Zootecnical Sciences (Opinion CINV.106/12).
RESULTS
There were higher values in the OFA 0.1 and 0.2% groups with respect to the control group during the egg lying period, showing significant differences (p<0.05) in the majority of weeks (Table 1).
Table 1 Effect of oligofructose from agave on egg weight (g).

ab Means with different letters in the same row are different (p<0.05).
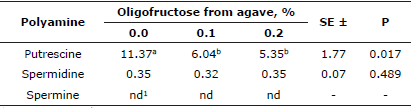
In the egg laying percentage of posture (Table 2), the control group and group with 0.2% OFA behaved similarly, their values were statistically superior with respect to the group added with 0.1% OFA from week 20 of egg laying.
Table 2 Effect of oligofructose from agave on egg laying percentage.
abc Means with different letters in the same row are different (p<0.05).
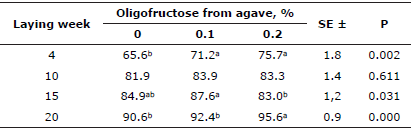
In connection with egg quality criteria, the highest values of average Haugh units were observed for eggs from the OFA treatment groups, which was statistically significant (p<0.05) at three of the four sampling points (Table 3).
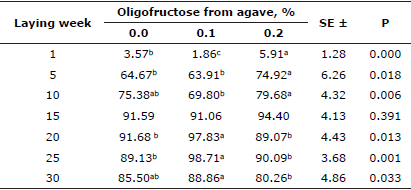
Table 3 Effect of oligofructose from agave on egg cholesterol values (in mg/100 g).
ab Means with different letters in the same row are different (p<0.05).
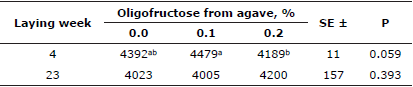
Egg cholesterol content determination at lying weeks 4 and 23 showed lower values for both groups of added OFA (Table 4) and significant differences (p<0.05) were found among treatments.
Table 4 Effect of oligofructose from agave on egg Haugh1 unit values.

ab Means with different letters in the same row are different (p<0.05).
Eggshell resistance results are shown in table 5. At week 4, eggs from the OFA 0.1% group had the highest shell resistance; this resistance was significantly (p<0.05) higher than that for eggshells from the OFA 0.2% group (Table 5). In contrast, at week 23, eggshells from the OFA 0.2% group had an increased resistance of 195 and of 177 g/mm2 compared to the OFA 0.1% and control groups, respectively. Nevertheless, all eggshells were classified as excellent condition.
Table 5 Effect of oligofructose from agave on eggshell resistance (in g of pressure/mm2).
ab Means with different letters in the same row are different (p<0.05).
With respect to fecal polyamine (Table 6), putrescine levels were significantly (p<0.05) lower in hen drops from the OFA 0.1% and 0.2% groups than those of the control group). Fecal spermidine levels did not differ significantly among experimental groups, while spermine was not detected in any of the samples.
DISCUSSION
Egg weight and laying. Hens from groups with OFA additives in their feed had significantly higher egg laying percentages and significantly higher egg weights compared to those of the control group. These results are consistent with previous reports [19], which determined that the addition of 1.0% oligofructose and 1.0% inulin to the diets for 4 weeks increased egg production by 13.35% and 10.30% respectively in comparison with the control group (p <0.05). In a previous study [19] an accumulated increase in egg weight was observed after the addition of oligofructose (1st week, 7.93, throughout the duration the experiment was 12, equivalent to 50%). In previous studies [8], noteworthy increases in egg laying percentage were observed in hens, with a better response in higher levels of inulin supplementation. It has also been reported that addition of a commercial oligofructose and inulin to the feed of laying hens increased both the percentage of egg laying and the weight of the laid eggs compared to those of the control group [14].
Egg quality. With respect to the Haugh unit value, OFA added groups exhibited the best results. This contrasts with results obtained by Chen y Chen [15] who observed no effect on the Haugh units of eggs laid to hens with inulin and oligofructose feed supplementation at several different temperatures and analysis times. However, in agreement with the present results, it has been reported an increase in Haugh units at different levels of inulin supplementation [8], and this effect was attributed to increased nutrient absorption and fermentation of beneficial bacteria as evidenced by the increased production of short chain fatty acids.
Eggshell resistance. The eggshell resistance did not differ between treatments and eggs were classified in terms of quality as "excellent" and "very good" according to the classification of Peebles and McDaniel [13]. On the other hand, it has been reported that the addition of oligofructose and inulin in the diet does not affect the shell characteristics [15]. It has also been observed that the inclusion of oligofructose in the diet of laying hens increases the concentration of calcium in the blood serum by 8% without effects on the characteristics of the shell [20].
Cholesterol. The cholesterol values agree with those found by Park and Park [8]. Also, these results are in agreement with that found by Chen et al [21], who reported that the addition of 10% inulin in diets of laying hens reduces the concentration of cholesterol by a percentage of 164%. The hypocholesterolemic effect of inulin demonstrated in the present study was not clearly understood. However, oligofructans produce short-chain fatty acids when fermented by microorganisms from the intestine, which contain mainly acetate, propionate and butyrate. In that sense Wolever et al [22] found that acetate increases total cholesterol and decreases fat.
Polyamines. The control hens showed putrescine accumulation values of 11.37 mol while 5.35 mol was found in the group added with 0.2% OFA. The general response to stress is activated via the second messenger and responds to the call to the intracellular program of stress. It shows, among other metabolic alterations, the transient increase of the polyamine metabolism called in 1992 as poliamyne-stress-response (PSR, acronym in English) [23]. This brain response implicitly involves the persistent accumulation of putrescine and an eventual reduction of spermidine and spermine. The values of OFA found in this work for putrescine agree with this theory. It has been postulated that a correct regulation of polyamine metabolism is the best response to stressors [24].
In conclusion, the use of OFA in the food reduced egg cholesterol values. It was demonstrated that there is a positive effect on the quality and weight of the egg with the addition of the OFA in the food of the hens.











 text in
text in