INTRODUCTION
According to the WHO, 60-65% of diseases that affect humans are zoonotic. Infants are most susceptible to infection through contact with dogs and cats while playing and coexisting with them; animals that roam freely or owners who are unaware of prevention plans may also result in infection [1,2,3].
Medellín, one of the cities with the highest growth rate (0.9%), had a population of 2,486,723 people in 2016; it is estimated that the pet population will increase proportionally with the human population, which is a determining factor in public health projects for the management, prevention, and control of zoonotic infections [4]. By 2015, 25.5% of the population kept dogs and 10.7% kept cats. Additionally, 50% of days live in the Metropolitan Area, where street dogs roam freely [1,4].
The gastrointestinal parasites of dogs and cats can cause diseases in humans, such as cutaneous larva migrans (CLM) or ocular larva migrans (OLM), or gastrointestinal disorders in children and adults [2,5,6]. Canine brucellosis, leptospirosis, and toxoplasmosis occur subclinically, unspecifically, or acutely in both animals and humans, leading to severe clinical symptoms [7,8,9,10]. Pets are the main mediators of contamination in areas of family recreation through the excreta, contact with animal secretions, or contamination of foodstuff [6,10].
The objective of the study was to establish the prevalence of these agents in companion animals in Medellín to contribute to the health of pets and lower the risk of zoonoses.
MATERIALS AND METHODS
Population and study area. The city of Medellín is located 1479 m above sea level and has a surface area of 380.64 km2, an average temperature of 22-24°C and a relative humidity of 63-73%. This is based on information from the Institute of Hydrology, Metrology and Environmental Studies (IDEAM) and the Mayor's Office in Medellín. The study is a cross-sectional study, in which apparently healthy dogs and cats were sampled on sterilization days in 2016; 1073 dogs and 428 cats were sampled for gastrointestinal parasites in the 16 communes and 5 townships that constitute the city, determined by means of the Quality of Life Survey [4]. The number of individuals sampled for Brucella canis, Leptospira spp., and Toxoplasma gondii testing was selected by convenience, and 500 dog and 500 cat sera were collected from 5 peripheral communes [1,2,3,8,13]. Information on species, sex, age, sector, socioeconomic level, and month of sampling was taken. The owners provided informed consent in advance for the use of the biological samples obtained.
Sampling. Stool samples were taken by rectal examination by prior lubrication with carboxymethylcellulose, then stored in a screw-cap bottle, preserved in 10% formaldehyde, and refrigerated at 4°C until its measurement. Blood samples were collected in MiniCollet® red cap tubes through the cephalic vein and then centrifuged at 1361 gx5 min for the separation of serum, which was stored in a 1.5 mL eppendorf tube at -20°C until processing.
Parasitological diagnosis. The microscopic examination of fecal matter included identifying the main causative agents of zoonoses [5]. This was performed with two techniques-direct examination with 0.85% saline solution and Lugol and the flotation technique with saturated saline solution, as described by Koffoyd and Barber [11]-to detect the presence of eggs or protozoa, such as ancylostomids, Toxocara spp., Dipylidium caninum, Giardia spp., and Trichuris spp.
Diagnosis of Brucella canis. The Rapid Plate Agglutination Technique was used with 2/3-Mercaptoethanol (PARP-2ME) [10]. Of the 500 serum samples, three from commune 3 were eliminated due to hemolysis.
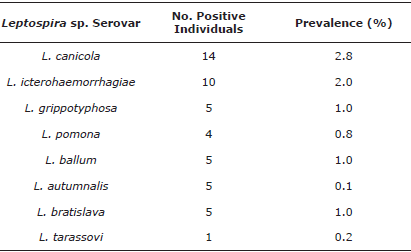
Diagnosis of Leptospira sp. This was conducted with the microagglutination technique (MAT), including the Canicola, Icterohaemorrhagiae, Grippotyphosa, Pomona, Ballum, Autumnalis, Bratislava, and Tarassovi serovars. The technique implemented and the cut-off point to determine seropositivity (antibody titers ≥1:100) were established according to the guidelines of the World Organization for Animal Health, as stated in the Terrestrial Manual of Leptospirosis, chapter 2.1.9, and by the Colombian National Institute of Health.
Diagnosis of Toxoplasma gondii. The Indirect Immunofluorescence Technique was implemented using the "Toxoplasma gondii IFA Feline IgG Antibody Kit" (Fuller Laboratories, CA, USA) following the manufacturer's instructions.
Statistical analysis. Excel 2016®, Epidat 3.1®, and SPSS Ver 22® were used to analyze the data to establish the associations and risk factors for species, age, sex, commune, level, sampling month, and sector, and the presence or absence of infection or titers of the diseases were investigated. After grouping the variables (Table 1), we considered the values p≤0.05, OR ≥1 or ≤1, and 95% CI as risk or protection factors, respectively.
Table 1 Independent variables.
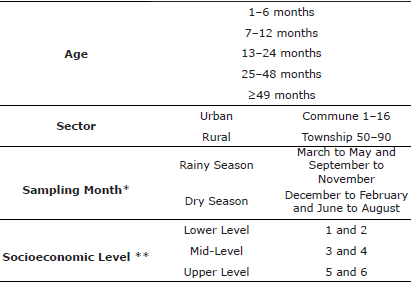
* Grouped based on the IDEAM.
** Classified based on the quality-of-life characteristics [4].
Ethical aspects. The blood and fecal matter samples taken during the neutering campaigns of the Mayor's Office in Medellín were obtained by trained veterinarians with the owner's prior informed consent and under the guidelines of Law 84 of 1989. The project has the respective endorsement of the Ethics Committee for Animal Experimentation of the University of Antioquia through Record No. 113 of October 12, 2017, and the authorization of the Secretary of the Environment of the Mayor's Office in Medellín.
RESULTS
Gastrointestinal parasites. Table 2 shows the findings on the prevalence of gastrointestinal parasites in dogs and cats as well as the prevalence for each individual agent.
Table 2 Frequencies of gastrointestinal parasites in dogs and cats by commune/township.
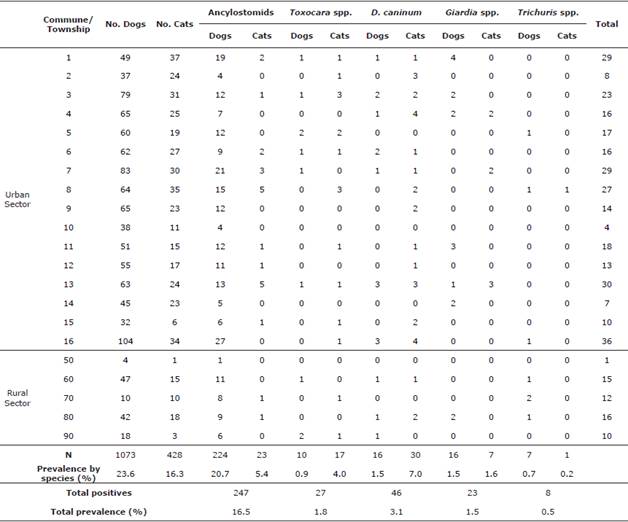
When making the comparative analysis between dogs and cats to determine which shows greater susceptibility to the same infections, it was found that cats have a protection factor when faced with ancylostomid positivity (p=0; OR=0.2). However, they represent a risk factor for positivity to Toxocara spp. (p = 0; OR=4.4) and D. caninum (p=0; OR=4.9). Dogs and cats aged 1-6 months (n= 1501) were found to be less prone to the ancylostomid infection (p=0.001; OR=0.5). The dry season posed a risk factor for the D. caninum disease for both species (n= 1501) (p=0.01; OR=10.7).
Table 3 Bivariate analysis of the factors associated with positivity to gastrointestinal parasites in dogs and cats. Evidence of risk or protective factor.
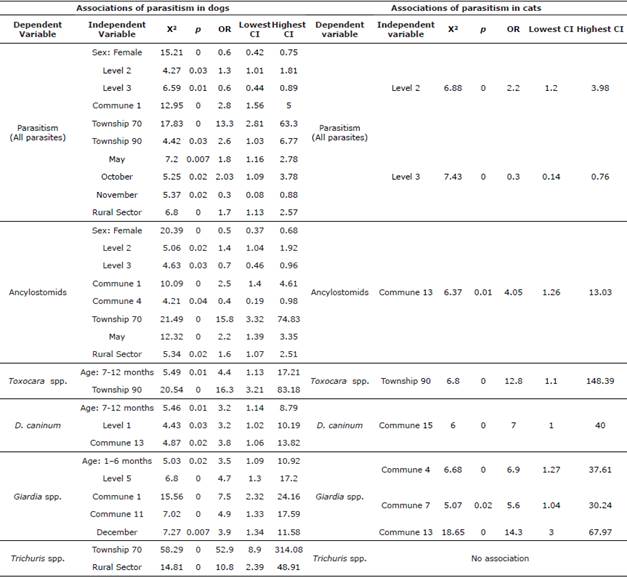
Note: Risk factor understood as p≤0.05 and OR≥1 or protection factor as p≤0.05 and OR≤1.
Brucella canis, Leptospira sp., and Toxoplasma gondii. Table 4 shows the seropositivity results in dogs and cats for B. canis, Leptospira sp., and T. gondii. Exposed individuals (≥1:20 and ≤1:160) and latent carriers (titers ≥1:320) are shown for the latter, which correspond to animals that are apparently healthy but harbor the agent, can disseminate it, and are also susceptible to reactivation of the infection [12,13]. Seroprevalence for the different serovars of Leptospira sp. is shown in Table 5; Table 6 shows the results of the multivariate analysis with the risk or protection factors associated with seropositivity for B. canis, Leptospira sp., and T. gondii.
Table 4 Frequency of seropositivity for B. canis, Leptospira sp. and T. gondii per commune.
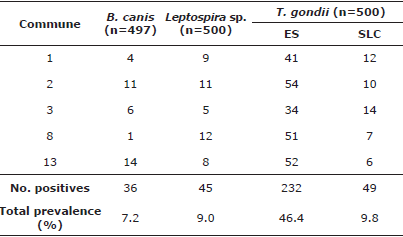
ES: Exposed Seropositivity (≥1:20 - 1:160); SLC: Seropositivity Latent carriers (≥1/320).
DISCUSSION
Gastrointestinal parasites. It is important to point out that dogs and cats throughout Medellín are positive for one or several agents, which make them parasite disseminators and a risk to public health due to interactions between humans and pets. They also represent a source of environmental contamination.
The frequencies of positivity in both dogs (23.6%) and cats (16.3%) are similar-the former was similar to what was reported in Colombia (19.7-88.6%) [1,5,14], whereas the latter was lower than what was previously reported (35-42.1%) [2,5]. This study was conducted on pets, which explains the low prevalence in both species. In addition, there has been a significant increase in the number of street dogs in Colombia, and ancylostomids are the most prevalent parasites [1,15].
The most prevalent agents in dogs were ancylostomids (20.7%), which are parasites that cause human CML. Their prevalence was similar with that found by other authors in Colombia (12.6-86.8%) [1,5,14] and Latin America (20-73.8%) [3,16]. This agent has been found in recreational areas for humans and pets [14]. The prevalence in cats reported in this research is lower (5.4%) than that reported in Colombia (7.4%) or Latin America (32.6-80%) [2,16]. Cats were also less susceptible to ancylostomids compared with dogs, possibly because of a greater restriction in going out and satisfying their physiological needs inside the home, thus accessing sources of infection to a lesser extent and decreasing the chances of them infecting humans or cross-infecting other cats.
In Colombia, a higher prevalence was reported for Toxocara spp. (37.2%) and Giardia spp. (10%) [2,5]. However, it is important to note that D. caninum represented a risk factor in cats (p=0; OR=4.9) and it showed a higher prevalence than dogs (7.0% vs. 1.5%, respectively). The Center for Disease Control and Prevention (CDC) highlights the importance of fleas in the process of transmission of D. caninum because they are infected by the cysticercoids of D. caninum and ingested by humans, or the pet may lead to the disease development. It is possible that the grooming habit of cats increases the risk of this parasitic infection due to the consumption of fleas. The infection mainly affects infants and preschoolers in close contact with pets, and they develop digestive conditions that may compromise their well-being [17]. Flea infestations must be controlled to ensure a complete prevention plan, both for the animal and for the human. Another study has shown a higher prevalence for D. caninum in cats (6.9%) than in dogs (2.2%) [3,14]. Internationally, prevalences vary in dogs and cats (1-88.3% vs. 2.8-81.6%, respectively) [2,3] and are higher than those reported nationally for both species (1.1-1.6% vs. 0%, respectively) [1,3,14].
CLM and OLM are caused by Toxocara spp. [2,3], which despite having lower prevalence in dogs (0.9%) and cats (4.0%) in this study than in those reported in previous studies in Colombia (2.5-11.8% vs. 5-37.2%, respectively) [1,2,5,14] and other countries (11-99.4% vs. 10%, respectively) [3,14,18], it is an agent of great importance for public health. The prevalence in humans is 3.8-91% in Colombia, with a seroprevalence of 63.3% in 30 individuals in Medellín, and the prevalence in Latin America is 2.5-63.2% [18,19]. Humans can become infected because a dog eliminates 1.4 million eggs a day on average into the environment, contaminating public parks and recreational areas [3], or due to the presence of infective eggs in the fur of pets [20]. Cats also represented a risk factor (p=0; OR=4.4) for toxocariasis, possibly related to the presence of latent eggs in the coat and their grooming habit or the consumption of paratenic hosts [14,19,20]. The Center for Food Safety & Public Health (CSFPH) of the United States highlights the importance of transmission in dogs and cats transparenterally, lactogenically, and through the consumption of paratenic hosts that harbor the larval stage of Toxocara spp. in their tissues.
Giardia spp. was not genotyped and it was not determined whether the protozoan found belongs to the genotypes of cross- or species-specific infection of G. duodenalis [ 5]. However, although the prevalence found for dogs and cats is low compared with what was previously reported in Colombia (0.8-16% vs. 14.8%, respectively) [2,3,14] and other countries (10-35% vs. 10-15%, respectively) [3,14], this agent produces cases of gastroenteritis in children aged under 5, with a prevalence of 13% in people and up to 33% in developing countries [3,5]. The results may be due to the fact that the water that supplies the homes of the urban area in Medellín is safe and that the hygiene and sanitation conditions of the city are good as it has a sewage system for wastewater, which lowers the risk of cross-infection. This is supported by the CDC and CSFPH, who have emphasized on the importance of consuming drinking water to reduce the risk of transmission of Giardia spp. In contrast, only this agent showed greater susceptibility in individuals from a high socioeconomic level. This may be influenced by access to sources of reinfection located just outside the urban area of Medellín, such as farms or towns where there is still no access to drinking water or sanitation, rivers, lakes, or puddles in recreational areas outside the city because dogs or cats living with owners from these levels have a greater possibility of being transferred together with their immediate family to other points where there may be more at risk of reinfection [3,5].
D. caninum and ancylostomids showed a risk factor in individuals of low socioeconomic levels, which can be related to reduced access to basic veterinary services and pet's semi-domestic habits, frequent in these levels, which contribute to greater exposure [3,5].
Age as a risk factor found in Toxocara spp. differs from that reported in other studies, which indicate a higher prevalence in puppies younger aged ≤6 months, since the main transmission mechanism of this parasite is lactogenic and placental [1,3,14]. The results found in the present study could indicate a possible reinfection or an absence of deworming in the tested individuals [14,20].
In the study conducted by Sierra et al [1], dogs were also found to have a higher prevalence of ancylostomids in the rural sector of Antioquia (87.1%) than in those living in the urban sector (24.1%). For Toxocara spp., more soil contamination is reported in urban areas than in sub-urban or rural areas [19], which was contrary to what was found in township 90. However, for Trichuris spp., there are no reports in the literature supporting the risk factor found in the rural sector.
In this study, dry months were a risk factor for the presence of D. caninum (p=0.01; OR=10.7) and Giardia spp. (p=0.007; OR=3.9), relating to what was found in Bolivia, where a greater prevalence was observed in both agents during the dry season [3,14,17]; while the wet months showed an association with positivity to ancylostomids (p=0; OR=2.2) and is supported by the literature [16,17].
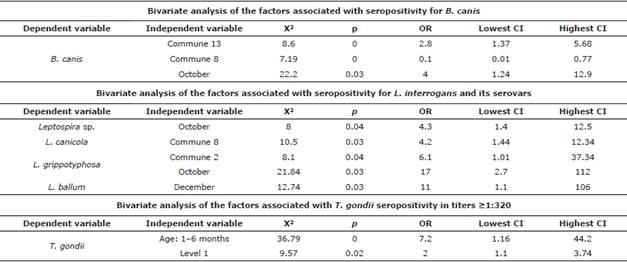
Brucella canis. This has been reported in Colombia since 2009; additionally, Castrillón et al [10] established that seropositivity in humans who own dogs for breeding purposes was 17% [7,10]. The prevalence of 7.2% mostly corresponds to unsterilized dogs without apparent symptoms. The most seropositive commune, and where a risk factor was found, corresponds to the one with more evidence of dogs without a permanent home or with more domestic-street dogs, according to the information provided by the inhabitants of the area. The months of the year with the highest seropositivity correspond to those with most rainfall in the region and represented a risk factor for seropositivity, possibly because rain favors the agent's permanence in organic matter, delaying its desiccation and increasing the probability of transmission through fomites and intake of contaminated material [10].
Leptospira spp. In Colombia, seroprevalence in men has been reported to be 12-62% [21]. Leptospirosis is a public health problem that emerged in urban areas due to the growth of cities and suburbs, displacement, and invasion areas [22,23], where the lack of basic sanitation, high rainfall, high infestations of rodents, and presence of stray dogs favor transmission [21,22]. Colombian cities have reported a canine seroprevalence for Leptospira sp. of 45% in the past, in addition to the reported transmission of leptospirosis from infected dogs to humans [22].
In Medellín, the presence of rodents implies different efforts by environmental health entities to establish the levels of infestation and the respective prevention and control measures. It may be possible that interspecies infection occurs because the serovars reported most frequently in this study are canicola and icterohaemorrhagiae, which are also frequently reported in humans in Antioquia (Sivigila) [8,22]. Canines can get contaminated from rodents, which are the reservoirs par excellence for L. icterohaemorrhagiae and L. ballum, found in different communes.
Dogs can be asymptomatic carriers of the disease, excreting large amounts of Leptospira for months or years after infection [24]. Additionally, there is a relationship between Leptospira sp., and flooding or high humidity areas [8]. Leptopira sp., survives for up to 350 days [24] under these conditions, and it can infect humans, susceptible dogs, and rodents, thus perpetuating the transmission cycle [8,24]. This may explain why the incidence of Leptospira sp. and L. gripotyphosa infections increase in October (rainy season) [8,21,22]. Considering the findings, it is necessary to implement vaccination plans for dogs to avoid their infection and subsequent transmission to humans [8].
Findings from this study may be due to the flaws or poor implementation of the vaccine prevention plan, because the only serovars included in the canine scheme are Canicola and Icterohaemorrhagiae [25], possibly due to the low socioeconomic level, low economic possibility to access vaccines, or population ignorance. Furthermore, the likelihood remains that dogs are exposed to other domestic or wild species that transmit different serovars that lack vaccines and in which, these rare serovars have been reported [8,25].
Toxoplasma gondii. Around 50-60% of pregnant women in Colombia have anti-toxoplasma antibodies (9), which indicate a fast circulation of the agent in the environment. Due to its tropical conditions, in Colombia, toxoplasmosis is considered a current disease with serious repercussions on pregnant women and neonates [9,13]. There is a potential risk that cats with high antibody titers (≥1:320) may eventually exit the latency period and initiate the elimination of T. gondii cysts; this is one of the many causes of transmission and would explain the high percentages of women seropositive to this agent [9,26]. Although cats are the definitive hosts, where the parasite's sexual cycle develops [9,13], it must be borne in mind that forms of transmission to humans do not require direct contact [9]. Despite the high seropositivity, there were no cats with active infections during sampling.
Seropositivity in cats owned by individuals of socioeconomic level 1, the one with the lowest quality of life, can be associated with little access to drinking water of both the human population and animals [22]. The risk posed by cats aged 1-6 months may be due to the transplacental and lactogenic transmission from the mother to offspring [27].Titers in adults indicate previous exposure, but no current infection or latency, and could be due to feral cat populations, in which higher seropositivity has been reported worldwide [9,13,26].
Final comments. This work demonstrates the importance of variables such as the socioeconomic level and the sampling area on the positivity for different agents of zoonotic origin of high and medium risk. It is important to raise awareness regarding the problem of zoonoses and its risk to public health in Colombia. Although Medellín has been a pioneer in animal welfare issues, relevant health plans for deworming and vaccination of companion animals must be established.











 text in
text in