INTRODUCTION
Cryptococcosis is a mycosis that affects immunocompetent and immunodeficient individuals, especially those with AIDS 1,2,3,4,5,6, its causative agent being the encapsulated yeast Cryptococcus spp., with its two pathogenic species C. neoformans and C. gattii; the first one has the varieties grubii (corresponding to serotype A, molecular pattern VNI and VNII) and neoformans (corresponding to serotype D, pattern VNIV), as well as the hybrid serotype AD molecular pattern VNIII. For its part, Cryptococcus gattii comprises serotypes B and C (molecular pattern VGI-VGIV) 7. In 2015 a taxonomic review of both complexes was published, with a new proposal of seven species: C. neoformans, C. deneoformans, C. gattii, C. bacillisporus, C. deuterogattii, C. tetragattii, and C. decagattii8.
The study of clinical and environmental isolates of Cryptococcus as a pathogen in humans is relevant in the Cúcuta region. The incidence of the disease in the general population in Colombia was 0.23 cases per 100,000 people, North Santander being the department with the highest incidence: 0.56 cases per 100,000 people. C. neoformans var. grubii, with the VNI molecular pattern, is the main etiological agent of cryptococcosis in both immunocompetent and immunosuppressed individuals; this mycosis has high morbidity and mortality and has been serving as a sentinel marker for HIV infection. 9 Epidemiological analyzes and the constant occurrence of clinical cases compatible with cryptococcosis show Cúcuta as a city with a high prevalence of infection, mainly that caused by C. gattii, molecular pattern VGII 10.
Taking into account the association of genetic subgroup VGII with high virulence and potential to generate outbreaks, this prevalence is worrying 10 Although infection with C. gattii is a minority in Colombia with respect to C. neoformans (serotypes A and D), the municipality of Cúcuta has a high prevalence (60%) in HIV negative patients 11 and globally, a lower frequency of cryptococcosis due to C. gattii is reported in immunocompromised patients including HIV / AIDS, which have been associated with genotypes VGIII and VGIV, as is the case of California, USA, considered an endemic area for VGIII, with infection attributed to it in 12% of these 12.
From the environment, serotype C of C. gattii was isolated for the first time in Cúcuta in 1998 13. Subsequently, in 2011 the first environmental isolation of C. gattii serotype B was obtained in this same city 9.
Regarding to the high incidence of cryptococcosis in the city of Cúcuta, the objective of the present study was to determine the presence of C. neoformans and C. gattii in the urban area of the city, and to establish the association of clinical isolates with those recovered from environmental samples.
MATERIALS AND METHODS
Study site and sample collection. We collected 1300 samples from 446 trees distributed as follows: soil (442), bark (434), nuts (40) and leaves (384); at the time of collection of the samples, the presence of feces of pigeons on their surface was selected as the main characteristic. The first sampling was carried out in October 2016 in the Mercedes Abrego, Simon Bolivar, Arcorirs (La Libertad) parks and peripheral zone to the General Santander Stadium. The second sampling was carried out in the month of January 2017 in the Parks Antonia Santos, La Victoria, Juana Rangel de Cuellar and Santander Park and the third, in the month of April 2017, was held in the National Parks and Fuente de Leones.
On the other hand, 6 isolates of C. neoformans cerebrospinal fluid (CSF) of HIV-positive patients with meningeal cryptococcosis were obtained from the Erasmo Meoz University Hospital of Cúcuta between June 2016 and June 2017. These samples corresponded to 4.3% of the total of those reported through the passive surveillance of cryptococcosis carried out in the country through the Epidemiological Survey on Cryptococcosis in Colombia.
Processing of environmental samples. A mapping of the sampling sites where each individual (tree) was identified was done to collect samples in each of the selected areas of the metropolitan area of Cúcuta, using plastic bags.
These were processed using a phosphate buffered saline (PBS) extraction technique and supplemented with antibiotics as previously described 14,15, for which 5 g of sample was weighed, resuspended in 25 ml of 1X PBS for homogenization and allowed to rest for 60 minutes; each homogenate was subsequently filtered with sterile gauze, and an antibiotic was finally added (allowing it to act for 60 minutes).
A total of 100 μL of each preparation was planted on agar (Guizottia abysinica) and then incubated at a temperature of 27°C for 20 days, with weekly periodic observations as previously described 16.
Creamy elevated colonies with regular edges and brown pigment (the production of melanin) were obtained and observed under a microscope using India ink (Figure 1). Every colony, corresponding to large round yeasts with the presence of a capsule, was subjected to urea degradation tests 17 and determination of species using CGB agar (canavanine agar glycine blue sodium bromide) 18.
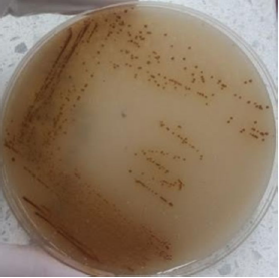
Figure 1 Cryptococcus sp. Agar culture on Guizottia abysinica agar (production of melanin). University of Santander, Cúcuta.
Processing of clinical isolates. An agar culture (Guizottia abysinica) was carried out for isolates of clinical origin (Figure 2) with urea degradation tests 14 and determination of species in CGB 18.
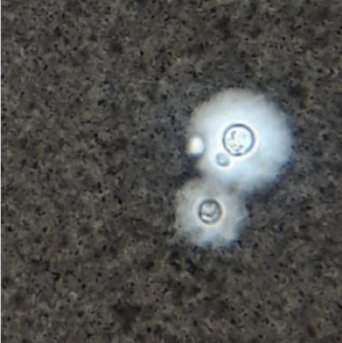
Figure 2 Cryptococcus sp sample of cerebrospinal fluid in India ink. Microbiology Group, National Institute of Health.
Determination of the molecular pattern by PCR-Fingerprint. The high-molecular-weight DNA was extracted using the phenol:chlororofm:isoamyl alcohol method according to Casali 19. This procedure was carried out using biomass obtained from the clinical and environmental strains once cultured on Saboureaud agar at 27°C for 48 hours. Subsequently, colonies were transferred from the culture to 1.5 ml microcentrifuge tubes and incubated at -20°C overnight. Then suspended in 500μL of extraction buffer (50mM Tris-HCl, 50mM EDTA, 3% sodium dodecyl sulfate, 2% mercaptoethanol), vigorously vortexed and incubated at 65°C for 1 hour. The lysate was extracted with phenol-chloroform-isoamyl alcohol (25: 24: 1, vol / vol / vol). The DNA was recovered by precipitation with isopropanol at -20°C overnight, washed with 70% ethanol (vol/vol) and then finally resuspended in TRIS-EDTA buffer (TE). The concentration was measured by fluorescence (Quibit 3.0 fluorometer).
The molecular characterization of the species of Cryptococcus spp, was carried out by PCR fingerprint, using as the first single specific sequence microsatellite (GTG) 5 (5'-GTGGTGGTGGTGGTG-3') described by Escandón et al in 2006 20.
The 50 μL PCR mixture contained: 31.5 μL of sterile deionized water, 25 ng of DNA, 5 μL of 10X PCR buffer (10 mM Tris / HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl) (Invitrogen , Carlsbad, CA), 0.02 mM of each dNTP (dATP, dCTP, dGTP and dTTP) (Promega, Madison, WI), 3 mM sodium acetate (Sigma, Atlanta, GA), O.8 ng of primer (for GTG5 the concentration was 10 ng), 2 mM MgCl, 1 ml of bovine serum albumin (BSA, 200 mg mL-1\Δ1) and 0.05 U Amplitaq. PCR was performed for 35 cycles in a SimpliAmp ™ Thermal Cycler using the following conditions: denaturation at 94°C for 20 seconds, annealing at 50°C for 1 minute, extension at 72°C for 20 seconds and a final extension cycle at 72°C for 6 minutes. The amplification products were separated by electrophoresis in a 1.4% agarose gel in Tris-borate EDTA (TBE) 1X buffer at 100 V for 2 hours. The products of the (GTG)5 amplification were stained with Gel red Nucleid Acid Gel Stain Biotium at 0.3 mg mL-1, for 30 minutes. The bands were visualized under UV light using 1 kb molecular size marker in three wells to allow the normalization of the gels. The molecular types (VNI-VNIV and VGI-VGIV) were assigned to compare the reference strains of the eight main molecular types loaded in each gel 21.
Determination of the molecular pattern by the RFLPURA5 gene and association analysis. The identification of the molecular types of Cryptococcus spp. both the clinical and environmental strains were performed with the two primers URA 5 (5’ATGTCCTCCCAAGCCCTCGACTCCG3 ‘) and SJ01 (5’TTAAGACCTCTGAACACCGTACTC3’).
The PCR reaction was carried out as described by Meyer et al 17; the PCR products were subjected to a double enzymatic digestion with Sau 96I and Hha I at 37°C for 3 hours. The restriction fragments were separated by electrophoresis in 3% agarose gel in Tris-borate EDTA (TBE) 1X buffer at 100 V for 5 hours. The restriction profiles were compared with the standards obtained from the reference strains, using analysis in the BioGalaxy module of the Biolomics program, version 3.0.
RESULTS
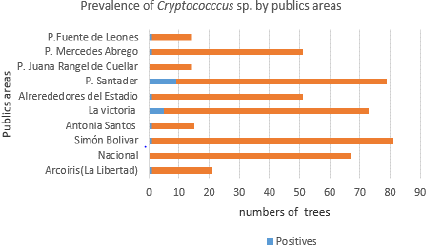
Characterization of environmental samples. A total of 75% of the trees analyzed came from 6 parks: Simón Bolívar Park with 80 individuals provided the most, followed by Santander, La Victoria, Nacional and Mercedes Abrego parks (with 70, 68, 67 and 50 individuals, respectively). This last number is the same for the area surrounding General Santander Stadium.
In turn, the diversity of the individuals analyzed in the present work comprised 10 species, with the Oití (Licania tomentosa) being the most widely distributed and predominant taxon with 66.4% of the total individuals, followed by the Almond tree (Terminalia catappa) with 13.2%, these two species comprising approximately 80% of the total sample. The remaining 20% was distributed among Mamoncillo (Melicoccus bijugatus) with 7.2%, Ficus (Ficus benjamina) with 5.4%, Tamarind (Tamarindus indica) with 2.9%, Chiminango (sweet Pithecellobium) and Totumo (1.8% each), Saman (Samanea saman), Lemon swingle (Swinglea glutinosa) and Mango (Mangifera indica) (0.4% each).
The initial culture revealed the presence of 21 isolates compatible with Cryptococcus spp., 20 of which corresponded to C. neoformans (95.2%) and 1 to C. gattii (4.8%), obtained from 20 of the 446 individuals, since double positivity was obtained for C. neoformans in the same tree (Table 1). Therefore, prevalences of 4.3% and 0.2% were obtained for C. neoformans and C. gattii, respectively.
Environmental isolates with their area of origin, species of the individual and isolation with molecular type identified by PCR fingerprint with primer (GTG) 5 and by RFLP URA5 gene are also described in Table 1.
Table 1 Description of positive samples for C.neoformans and C.gattii recovered in the city of Cúcuta, Norte de Santander.
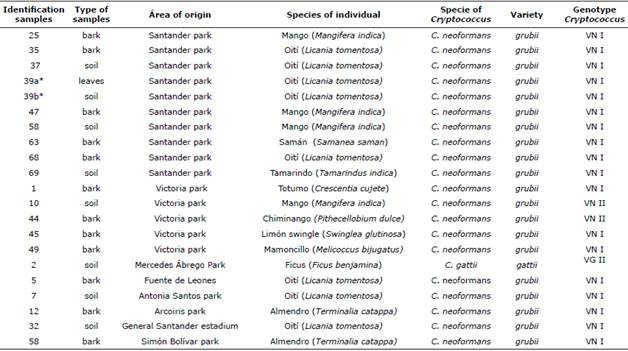
* Samples derived from the same individual.
In total, Santander Park occupied the first place in prevalence with 47.6% of the 21 isolates obtained, followed by La Victoria Park (23.8%) and finally Fuente de Leones, Antonia Santos, Arcoíris (La Libertad), and Simón Bolívar parks and the surroundings of General Santander Stadium (each with 4.8% of the isolates), exclusively for C. neoformans var. grubii, since a single isolate of C. gattii was obtained in Mercedes Ábrego Park (4.8% of the isolates).
At the same time, the calculation of the prevalence for each public area studied revealed outstanding data, especially for two of these: Santander Park with 12.8% (9/70 individuals were positive) and La Victoria Park with 7.3% (5/68 individuals were positive), in both cases only for C. neoformans.Figure 3 shows the individual prevalence for the different areas evaluated.
The positivity analysis according to the type of sample showed a predominance of C. neoformans isolates obtained from bark and soil with 60% (12/20 isolates) and 35% (7/20 isolates), respectively, while only one was obtained from leaves (5%). This implies a general prevalence for C. neoformans from bark, soil and leaves of 3.0% (of 434 samples), 1.6% (of 442 samples) and 0.3% (of 384 samples), respectively.
Regarding C. gattii, positivity was only obtained in a soil sample (Table 1). In general, no isolates were obtained from the 40 samples of nuts analyzed in this study.
The presence of C. neoformans VNI was concentrated in 55.5% in the Santander park (10/18 isolates), followed by 16.6% in Parque la Victoria (3/18 isolates) and a minority was distributed in the Fuente de Leones parks , Antonia Santos, Arcoíris (La Libertad), Simón Bolívar and surroundings of the General Santander Stadium, with 5.3% of the isolates for each zone (1/18 isolates), while the molecular type VNII was found exclusively in the Victoria Park, isolated from soil and bark of Mango tree species (Mangifera indica) and Chiminango (Pithecellobium dμLce), respectively.
On the other hand, the isolate of C. gattii corresponded to VGII, being in turn the only one obtained from the Mercedes Abrego park, from soil associated with an individual of Ficus (Ficus benjamina) (Table 1).
Characterization of clinical samples. Genotypic analysis from clinical samples revealed the presence of C. neoformans in all cases, corresponding in their entirety to the VNI molecular type (Figure 4).
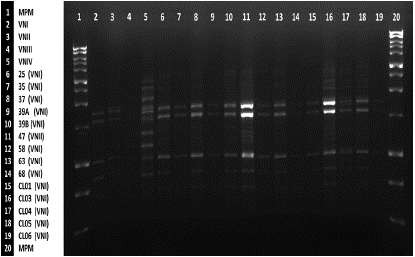
Figure 4 PCR fingerprinting using primer (GTG)5 from clinical and environmental isolates of Cryptococcus neoformans var. grubii with molecular weight marker 1kb and control strains.
Genetic relationship between C. neoformans isolates of clinical and environmental origin The PCR fingerprint and RFLP of the URA5 gene, revealed that the genetic pattern found in those isolates from patients is also present in most of the environmental isolates, being VNI molecular pattern the most prevalent. A correlation was observed between these two types of isolates at the genetic level/Santander park (10/18 isolates), followed by 16.6% in Parque la Victoria (3/18 isolates), and a minority were distributed among the Fuente de Leones, Antonia Santos, Arcoiris (La Libertad), Simón Bolívar parks and surroundings of General Santander Stadium, with 5.3% of the isolates for each zone (1/18 isolates); the molecular type VNII was exclusively found in Victoria Park from the soil and bark of the Mango (Mangifera indica) and Chiminango (Pithecellobium dulce) trees, respectively.
In contrast, the isolate of C. gattii corresponded to VGII, being in turn the only one obtained from Mercedes Abrego Park from an individual Ficus (Ficus benjamina) (Table 1).
DISCUSSION
In the ecological context, there has been a predominance of C. neoformans, especially C. neoformans var grubii VNI and VNII (serotype A), whose habitat also extends to bird excreta 22. These two molecular types are also the most prevalent in cases of cryptococcosis (97%) in immunosuppressed individuals in Colombia 21,23 and in other countries, including Europe 24. However, the environmental presence of C. gattii is particularly relevant to its ability to generate disease in immunocompetent individuals and its potential for adaptation to different geographical conditions, ceasing to be exclusive to tropical and subtropical areas, to also be found in temperate and rainy areas 25.
The present work covered 10 highly attended public areas of the city of Cúcuta, in which most of them revealed the presence of pathogens of the genus Cryptococcus sp, except for the National Parks and Juana Rangel de Cuéllar. The prevalence found was, in consistence with the previously mentioned 22, higher in general for C. neoformans (4.3%) with respect to C. gattii (0.2%) at a ratio of 21.5: 1, but on the other hand, contrasted with previous data reported for the city of Cúcuta in particular (years 2008 and 2009), having been referred to as 0.07% in a similar way for both species of the fungus, even with a significantly greater screening of individuals (3634) from La Libertad, San Eduardo and around the General Santander Stadium 26.
According to our findings, the positivity for C. neoformans showed predominance in the Santander park (47.6% of the 21 isolates), followed by the La Victoria park (23.8%) and finally the parks Fuente de Leones, Antonia Santos, Arcoíris (La Libertad) , Simón Bolívar and surroundings of the General Santander Stadium (each with 4.8% of the isolates), exclusively for C. neoformans grubii variety. Taking into account the above, we can conclude that the Santander parks (especially with individual prevalence greater than 10%) and La Victoria, constitute relevant ecological niches for C. neoformans, the latter also providing genetic variability since both the VNI molecular type is found the VNII (only area with finding of the latter).
There was a variety of arboreal species that were found to be niches of the fungus, especially Oití (Licania tomentosa), widely distributed in the areas evaluated, from which 38% of the total isolates were identified (8/21 isolates), all corresponding to C. neoformans VNI, besides being in diversity of sources (bark, soil and leaves), followed by Mango (Mangifera indica), from which 4 isolates (19%) were obtained, both on soil and in bark , corresponding to C. neoformans variety grubbii (VNI and VNII) and the Almond tree (Terminalia catappa), with two isolates (9.5%), identified as C. neoformans VNI, in contrast with the previous report of the latter as habitat of both C. neoformans as of C. gattii25.
In Santander Park interestingly, the five isolates obtained from the Oiti tree species (Licania tomentosa) corresponded to four individuals, since one of them was double positive, revealing the presence of C. neoformans VNI simultaneously in soil adjacent to the same and in its leaves.
This unique case of double positivity for Cryptococcus neoformans, recorded in the present work has no antecedent in the city, as regards the type of tree (Licania tomentosa).
According to the data published by Firacative et al 26, the tracking of more than 4300 samples from trees from different zones of Cúcuta and areas near the housing of patients infected with C. gattii serotype B (VGI / a), yielded only one isolate of this pathogen, two isolates of C. gattii serotype C (VGIII/a) and three isolates of C. nooformans serotype A (VNI/ a), where one of the latter, together with the only C. gattii isolate (VGI / a) were obtained from the same tree but in that case from Ficus species (Ficus benjamina) in the San Eduardo sector.
Similarly, around the General Santander Stadium, also on the soil of the same Ficus tree (Ficus benjamina) were C. gattii (VGIII / a) and C. nooformans serotype A (VNI/a) simμLtaneously isolated 26.
In relation to the above, the present work revealed positivity for this last species of tree, but corresponding to the isolation of C. gattii VGII from soil sample in the Mercedes Abrego park (without antecedent), in the heart of the downtown area of the city from Cúcuta. This was the fourth area with the largest number of individuals screened, however, only one of its 50 trees was positive, being an individual Ficus (Ficus benjamina).
Ficus benjamina, harboring both C. gattii and C. neoformans26, is important if the presence of VGI, VGII and VGIII genotypes is considered in both environmental samples and in patients with cryptococcal disease (10,21, 23). These three molecular types have also been associated with cases of human and animal cryptococcosis in the western United States. Particularly the genotypes VGII and VGIII of environmental origin have been identified as the possible etiological agent of mycosis in HIV positive individuals 27.
The findings in the area surrounding the General Santander Stadium are consistent with a previous report, which showed the presence of C. neoformans26, allowing to conclude about its persistence in the area. However, this positivity was observed for the first time in trees of the species Licania tomentosa, reflecting a variation of both the types of habitat of the fungus, and of the species of the pathogen circulating in a given area.
Given the wide environmental distribution of C. neoformans and C. gattii, and their association to pathology in immunosuppressed and even immunocompetent individuals, the permanent tracking of common areas of high population confluence is relevant 25.
In agreement with the above, the recognition of the identity between isolates of Cryptococcus spp from clinical to environmental sources, by means of the comparison of circulating genotypes is of great interest taking into account also the increase of cases of cryptococcosis in HIV positive individuals, comparing the time periods 1997-2005 with the period 2006-2010 (3000 cases/ million inhabitants and 3300 cases / million inhabitants, respectively) 21,23. In addition, the incidence of cryptococcosis also affects minors in Colombia (under 16 years of age), where HIV infection is a recognizable risk factor in only half of the cases, and additional factors remain to be established. It is worrisome, however, that this incidence is seven times greater for the same population group in Norte de Santander (0.122 cases/100.000 inhabitants), with respect to the national average (0.017 cases/100.000 inhabitants) 28.
The genotypic analysis of the pathogenic species from 6 clinical samples in the present work revealed exclusively the presence of C. neoformans var grubii VNI (100% of cases). Consistent with the broad prevalence registered for this in studies such as González et al 29 in Mexico, over 12 years, from 166 clinical samples (it is not specifically specified if HIV + patients), who reported prevalence for C. neoformans of 92.2%, with respect to C. gattii (7.8%) and corresponding to VNI (74.6%), VNII (9%), VNIII (4.8%), VNIV (3.6%), VGI (3.6%), VGII (1.8%), VGIII and VGIV (1.2% each) 29.
It is necessary to expand the number of clinical isolates that allow determining associations with the environmental isolates of the VNII pattern, as well as C. gattii present in the environment.
It is noteworthy that Cúcuta is characterized by widely forested areas and the presence of a large number of birds, mainly highlighting the Columbia livia pigeon in the parks studied, being more representative the presence of these in the Santander Park where this work allows to confirm the yeast niche, other studies also show the relationship between ecological niches and these birds 30, hence the importance of a epidemiological surveillance due to the proximity to humans and the fact that they can move from a park to the other.
These data related to the high prevalence of patients with a diagnosis of cryptococcosis and its genetic relationship with environmental isolates is of great importance in public health programs.











 text in
text in