INTRODUCTION
West Nile virus (WNV) is an RNA virus of the Flaviviridae family, positive-sense single-stranded, whose transmission vectors are mainly mosquitoes, WNV has been the main cause of arbovirus encephalitis worldwide, which forms part of the Japanese encephalitis complex 1 The virus was first isolated from Uganda in 1937; later years caused epidemic outbreaks in Asia, Europe and Australia; and in 1999 it appeared for the first time in the American continent in the city of New York (USA) 2. The WNV genome is 10.8 Kb in lenght, which is translated and processed into 10 proteins: 3 structural proteins (envelope [E], matrix [M], capsid [C]) and 7 non-structural proteins (NS) (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) 3,4,5. Exocytosis is the mechanism by which vesicles loaded with virions emerge from the host cell; these virions are assembled in the endoplasmic reticulum 6,7,8.
The WNV is considered an avian-type zoonosis, which circulates and is maintained in nature through an enzootic cycle between avian species and mosquitoes. The species Culex pipiens, Cx. tarsalis and Cx. quinquefasciatus are the most important vectors in the transmission of the virus in North America 9.
Most human infections are asymptomatic, approximately 20% of infected people become symptomatic and develop acute diseases, ranging from a systemic flu to neuroinvasive outcomes (meningitis) 10; in animals the affected species are equines, which in the worst case can generate a fatal neuropathy 11.
Currently, it has been proven that the circulation of WNV in nature occurs through a transmission network (many cycles) in which it involves some genera and species of birds, some genera and species of mosquitoes, some mammals and ticks 12.
Human beings infected with WNV can present a mortality rate between 1 to 30% 12, in contrast, the death rate in equines ranges between 38-57.1% 13.
Since entering the American continent, viral activity has been reported in equines and birds in most countries of the continent 14. The first case of WNV in South American ecosystems occurred in 2006 in Argentina, where the virus was isolated from the brains of two dead horses 15, in addition, serological cases of horses have been recorded, in Trinidad 4% 16, Colombia 9.23% 17, Venezuela 4.3% 18, Argentina 16.2% 19, Brazil 3% 20 and Uruguay 2% 21.
On the other hand, all flaviviruses are antigenically related, giving frequent cross-reactions, especially in places where two or more flaviviruses are endemic and enzootic, so it is meritorious to be confirmed by different diagnostic methods 11. Regarding serological studies for WNV as a screening test, the ELISA serological test is recommended, but it is necessary to confirm the samples, through the Plaque Reduction Neutralisation Test (PRNT) due to the intense cross-reactions between flaviviruses 22.
In Ecuador, investigations have been carried out in different sites to detect the presence and circulation of WNV; besides that, different avian species have been searched, both domestic and wild, inside and outside the continental Ecuador. Among the Ecuadorian ecosystems to be highlighted are the Galapagos Islands 23,24,25, the Gulf of Guayaquil 25, Jauneche and the Abras de Mantequilla wetland in the province of Los Ríos 26.
Studies conducted in Ecuador: Study Sites
Galapagos Islands. It is an archipelago located 972 km off the coast of Ecuador, declared a World Heritage Site by UNESCO, is composed of 13 large islands, 6 medium islands and 215 islets, and is very famous in the world for its biodiversity 25. In august 2013, in the Isabela and Fernandina islands, a serological investigation against WNV was conducted in 69 endemic birds called flightless cormorant (Phalacrocorax harrisi). 23; also, between 2003 and 2004, on the same islands described above, a serological study was carried out on 195 penguins (Spheniscus mendiculus) 24, in addition, during October 2008, May 2009 and November 2010 in the islands of Santa Cruz and Baltra, and during February 2010 in San Cristobal Island, in 423 live birds (domestic and wild) serological studies were carried out, and in 156 birds Viral identification studies were performed (RT-PCR)25.
Guayaquil. It is an eminently tropical city, of great tourist importance that is located in the Gulf of Guayaquil. Studies were conducted in January 2011 in three areas of Guayaquil: north (Capeira-Pantanal), southeast (Fundación Ecológica Andrade) and center (Guayaquil Historical Park), which covered a range of habitat types (private gardens, parks, urban areas, mangroves y forests) multi-diverse; in these sites 202 domestic and wild birds were sampled for serological studies, but there were not determined cases of WNV 25.
Jauneche Sector. It is a small area located in the northern part of the Province of Los Ríos, bounded on the north by the canton of Mocache, Palenque to the southwest and the Peñafiel estuary to the east; in this mentioned sector there is a reserve of 130 hectares where the scientific station "Pedro Franco Dávila" is located, the same one that is directed and protected by the University of Guayaquil. In the above-mentioned sector between 2007 and 2009, 20 horses with ages between 3 to 7 years were sampled 26.
"Abras de Mantequilla" wetland. Is a RAMSAR site of national and international importance, which borders the provinces of Guayas, Manabí, Bolívar, Cotopaxi and Pichincha; its jurisdiction is made up of Vinces, Pueblo Viejo and Baba cantons. It belongs to the tropical sub-humid forest and has a total area of 67.177 hectares. Its geographical coordinates are 1°28'00 "south latitude and 79°35'00" west longitude; In addition, the average annual temperature is 25°C, the average humidity is 82% with maximums coinciding with the warmer months and the annual rainfall is 1260 mm 27.
In the Abras de Mantequilla wetland, two studies were carried out and in both the animals were selected according to the following inclusion criteria: equines belonging to the research area or with a stay greater than 21 days and older than 3 months of age. It should be noted, that the animals in question had an owner and were not wild horses.
In addition, a survey was conducted, where data were recorded such as identification of the animal, breed, sex, age, symptoms, vaccination and transfer of animals to other areas.
It is important to note that only in the first study serological studies were performed in 9 people, whose ages were between 40 and 60 years of age. The number of equines sampled in the wetland in the years 20072009 was 160 26.
Sample collection. A total of 630 animals were counted, and a guided sampling was conducted according to the consent of the equine owners who wished to participate in the study. In the first study the samples were taken between September and December 2007, obtaining a total of 160 samples of equine blood serum and 9 human samples, from 5 sectors of the wetland that are: La Piedad, La Luz, Los Playones, Jobo and Mapancillo. In Jauneche 20 samples of equine blood serum were taken.
Regarding the second work (Abras de Mantequilla), the samples were collected between January 5 and December 22, 2012, obtaining only a total of 412 equine blood serum samples that were collected in the aforementioned sectors 36.
The selection criteria of the areas studied was based on ornithological characteristics, for the first study (places where migratory birds arrive most, there is no hunting presence and food availability), and epidemiological, for the second study (presence of cases).
It is important to mention that the residents of the Abras de Mantequilla, in both studies, were told about the importance of the study and the risk that arboviruses has for the environment; in addition, the people who participated in both studies were asked to give their informed consent. On the other hand, prior to taking the sample in the horses (in the two studies), informed consent was requested from their respective owners.
In equines the sample was extracted from the jugular vein, and in humans from the humeral vein (at the elbow flexure), then they were transported at a temperature between 4 to 8°C to the Virology Laboratory of the National Institute of Hygiene and Tropical Medicine "Leopoldo Izquieta Pérez" (INH-MT "LIP") of Guayaquil, to search for antibodies against WNV.
The INH-MT "LIP" during the samplings did not have a bioethics committee, but it is meritorious to highlight in the ethical and legal aspect, that the name of the people submitted to the study were not published nor their data disseminated. Moreover, the owners of the animals were handled properly and no drug was administered; furthermore, it was reported that if the diagnosis was positive, it would be informed immediately to adopt prevention and control strategies. In the same way, we proceeded with the equines of the study.
On the other hand, it is worth mentioning that the areas studied were not easily accessible, mountainous, with many lagoons and wild animals.
Laboratory analysis. The serum was obtained by centrifugation, then transferred to 2 ml vials and stored frozen at -20° until analysis. The techniques described by the OIE for the detection of WNV are: real-time RT-PCR, isolation in tissue culture, y IgM capture (IgM capture ELISA), Plaque Reduction Neutralisation test (PRNT), serum neutralisation and immune-histochemistry 28. In the first study, the samples were analyzed with the PanBio® West Nile Virus IgM Capture ELISA diagnostic kit and were confirmed by PRNT; on the other hand, for the second study the samples were studied by the blocking ELISA test and were also confirmed by PRNT.
Laboratory Techniques. PanBio® West Nile Virus IgM Capture ELISA: The components of this test are: recombinant antigen (NY 99), conjugated (peroxidase conjugated monoclonal antibody WNV), substrate (tetramethylbenzidine and hydrogen peroxide) and stop solution (phosphoric acid) 29.
Blocking ELISA test: The technique was optimized with NY 99 antigens (FOCUS diagnostics brand), anti-Flavivirus conjugated with horseradish peroxidase, ABTS substrate y stop solution (Sulfuric acid) 22,30.
Plaque Reduction Neutralisation test: The first study was conducted at the Center for Disease Control and Prevention (CDC) of Puerto Rico with the following procedures: The samples were heat-inactivated at 56°C for 30 minutes, then double serial dilutions were made in phosphate buffered solution (PBS), with 30% heat-inactivated fetal bovine serum. The sera were diluted 1:20 with diluents (Chimera Vax WNV); also used VERO cells, NY 99 virus, media containing 10% of M199 without phenol red, 1% of essential amino acids, 1% of vitamins, 1% glutamine, FBS inactivated to 5%, gentamicin to 0.4%, sodium to 4%, bicarbonate, 0.6 of agarose and the stain was made with neutral Red 31.
The second work confirmation of the samples was performed in the arbovirus laboratory of the Virology Institute "J. M. Vanella" from the University of Córdoba (Argentina). This test used: VERO cells, strain WNV E / 7229/06, dilutions were made from 1:10, but were considered as positive from 1:20 dilutions 15,32.
Results obtained from the WNV search in Ecuador. From the serological and viral investigations of WNV carried out in 2003, in 69 endemic birds from the Galapagos Islands 23, besides, between 2003 to 2004, in 195 penguins 24, and those carried out during October 2008, May 2009, November 2010 and February 2010 in the San Cristóbal Island 25, on live and dead birds; there were no virus case determined on such species aforementioned. In the city of Guayaquil neither WNV case were evidenced 25; and at present there has no case evidence reported, from the Ecuadorian health control organisms (Ministerio de Salud Pública and Agencia de Regulación y Control Fito y Zoosanitario), nor from the particular or institutional researchers.
On the other hand, in Jauneche from the 20 equines sampled no serological evidence of WNV was detected, contrasting with the "Abras de Mantequilla" wetland study, where from a total of 160 horse serum samples analyzed, 13 were reactive (8.12%) and from 9 humans, 2 were reactive (22.22%) for IgM antibodies type against the WNV through the Elisa technique, developed in the Subprocess Virology Department of the National Institute of Hygiene and Tropical Medicine "Leopoldo Izquieta Pérez" (INH-MT "LIP") of Guayaquil. The reactive samples were analyzed by NTRP in the CDC of Puerto Rico, to confirm the presence of reactive antibodies, finding a total of 5 confirmed cases in equines (3.12%) and none in humans (Table 1).
Table 1 Study conducted on equines and humans from two selected areas of Ecuador 26.

Fuente: Coello Peralta RD, Diaz Castillo A, Medrano JB. 26.
The 5 seropositive samples corresponded to horses from the La Piedad sector with two cases in males, five and seven years of age; La Luz with a male case another in female, with ages of 2 to 6 years respectively; and a two-year-old male Mapancillo animal.
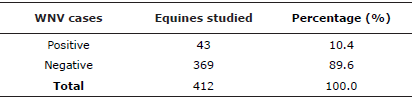
From the 412 samples analyzed in the second study, 52 were reactive (12.6%) for IgM antibodies against WNV by the blocking ELISA technique in the Virology Laboratory of INH-MT "LIP" of Guayaquil, the reactive samples were confirmed in the arbovirus laboratory of the Virology Institute "J. M. Vanella" from the University of Córdoba (Argentina), 43 confirmed cases were determined, which allowed to determine a prevalence in horses of the "Abras de Mantequilla" of 10.4% (See table 2).
The positive samples (confirmed) corresponded to horses from the 5 selected areas of the "Abras de Mantequilla" wetland, presenting in La Piedad 9 cases, 6 males and 3 females; in La Luz 7 cases, 4 males y 3 females; in Los Playones 10 cases, 7 males and 3 females; in Jobo 8 cases, 4 males and 4 females; and in Mapancillo 9 cases, 6 males and 3 females. The aforementioned animals were of mixed race and did not have vaccination history. It is important to note that the final prevalence recorded in the Abras de Mantequilla was 6.76% 26,36.
On the other hand, in the first study conducted in the "Abras de Mantequilla" wetland, 3 animals presented symptoms similar to West Nile Fever, in the second study there were no symptoms, in addition, in the first study with respect to sex, males presented 56% and females 44%; in the second study, males showed 60% and females 40%. But, in the two studies they coincided with respect to the ages (between 3 months to 12 years), mixed race, with no vaccination history and 90% of the owners of the animals said that their horses if moved to different sites 26.
DISCUSSION
The WNV is one of the most widely distributed flaviviruses in the world and with emerging activity in the Americas, since 1999 33.
The absence of viral activity in Galapagos, Guayaquil and Jauneche is presumed by the non-introduction of infected or diseased migratory birds of great ecological impact of Ecuador and the world; On the other hand, there are no publicly updated data on the search for antibodies in serum from mammals (humans, equines) or birds.
The 3.12% and 10.4% of serological evidence obtained in the first and second studies respectively, determined a seroprevalence of 6.76%, in equids of the "Abras de Mantequilla" wetland of Ecuador, the same one that is among the seroprevalence parameters reported in equines from Latin America (1 to 16%) 19, using the same laboratory techniques (ELISA and NTRP), in addition, the confirmed antibodies (IgM) indicate recent infection, which evidences the permanent activity of the WNV in said zones.
In South American countries seroprevalences are registered in equines of 5 and 9% in Colombia 17,34; Venezuela 4% 18; Brazil 3% 20 y 8% 35; Uruguay 2% 21 and Argentina 16% 19 (See table 3).
Regarding the gender, races and ages studied in equines, the scientific literature indicates that there is no predisposition to become infected with WNV 33.
In addition, the animals studied had no history of vaccination, but 3 animals (from the first study) showed symptoms similar to West Nile Fever in equines; with fever, weakness, decay and prostration, these same animals were reactive and positive for WNV, but they did not die. These animals are reliable proof that the virus in Ecuador is circulating unnoticed.
Besides, the zones of the Abras de Mantequilla are sites of great wild impact since there is a great variety of ecosystems, where a great diversity of migratory birds arrive, which could lead to a serious problem of wildlife, animal and human health; In addition, the studied zones are routes that birds follow in their migration during the cold season in the North American countries, which constitutes a risk factor for the transmission of various arboviruses 26,27.
In conclusion, the presence and circulation of WNV in equines of Ecuador was determined, human cases were not confirmed (inconclusive). From the point of view of animal and public health, the site where the serological presence of the virus was found, is an ecological site at national and international level of high impact, due to the high number of migratory avian species; additionally, it is concluded the clearly risk for wildlife, animal health and could become a serious public health problem, since the virus can cause meningoencephalitis in horses and humans.











 text in
text in