INTRODUCTION
Marek's Disease (MD) is a global neuropathic and lymphoproliferative disease in chickens (Gallus domesticus) caused by a highly contagious oncogenic alphaherpesvirinae [ 1,2. Its genomic organization and molecular structure are similar to the human Herpes Simplex Virus (HSV) of the alphaherpesvirinae subfamily [1,3]. However there are some unique features that differentiate Marek's Disease Virus (MDV) from other Alpha-herpesvirus: it establishes latency in lymphocytes (T-cells), it is strictly cell associated, encodes for an oncogene (Meq), and is able to induce CD4+ T cell lymphomas [3,4]. The disease is characterized by immunosuppression, infiltration of infected lymphocytes into peripheral nerves and visceral organs (liver, kidney, spleen, gonads, heart and proventriculus) and ultimately the formation of metastatic T-cell Lymphomas [5,6].
Affected unvaccinated and susceptible chickens will usually succumb to the disease, resulting in high morbidity and mortality [7]. Due to research in the early 1970's, the control of the disease was achieved with the introduction and widespread use of the non-pathogenic strain Meleagrid herpesvirus 1 (Herpesvirus of turkeys or HVT). Over the years, a decreased in vaccine effectiveness emerged due to interference with maternal antibodies and appearance of new highly virulent field strains of MDV [8]. It is believed that MDV evolution towards higher pathogenicity was caused by high density housing, genetically inbreed lines, improper vaccine handling, exchange of breeding stock animals and little knowledge of proper hygiene practices (Figure 1) [9].
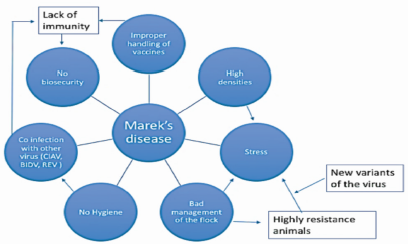
Figure 1 Causal web of Marek’s disease. Outbreaks of MD from lack of immunity presumably occur from improper handling of vaccines, stress generated by high density housing and inadequate management practices, lack of biosecurity, poor hygiene, infection with new variants of the virus (vv+MDV), and coinfection with other immunosuppressive viruses.
MDV can only be transmitted horizontally, so disease control can be achieved through implementation of strict biosecurity procedures, management practices, genetic selection and rigorous vaccination programs [2,4,5].
In the case of MDV, control programs should be based on knowledge of current pathotypes or virus strains present in the farm, dynamics of infection, and evidence of involvement in the production scheme [5,8]. Therefore, it is crucial to have diagnostic tools capable to characterize circulating field strains and monitor the release of the vaccine virus into the environment. Although there is some information about MDV in Colombia, the dynamics of the viruses that are currently circulating in poultry farms and backyard chicken, as well as the pathotypes present in the field is scarce. This review will focus on the current situation of MD in four Colombian farms, new diagnostics tools available in the country, and some recommendations for control in case of clinical disease.
Etiological agent of Marek's disease. MD is caused by an oncogenic Gallid herpesvirus 2 (GaHV-2), also known as MDV-1 or serotype 1. Herpesviruses are enveloped viruses with an icosahedral capsid. MDV specifically is a double-stranded linear DNA virus of approximately 175 kb in length that codes for 103 proteins (Figure 2), some of which are associated with oncogenesis [3]. GaHV-2 is strictly cell-associated in all organs except in feather follicle where infectious virions are shed into the environment as part of dander and dust [2]. Chickens are susceptible to infection by MDV, but some genetic studies suggest that specific major histocompatibility complex (MHC) haplotypes may confer resistance to disease progression that prevents neuropathy and lymphoma formation [10].
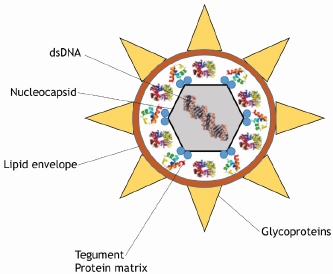
Figure 2 Schematic representation of an Alphaherpesvirus. The linear-double stranded DNA (dsDNA) is enclosed within an icosahedral nucleocapsid, which is surrounded by tegument proteins (proteinaceous matrix) and the lipid envelope containing several viral glycoproteins (gB, gC, gD, gE, gH, gI, gK, gL, and gM) [5].
The first case of MD was published in 1907 by Dr. Joseph Marek in Hungary, after whom the disease is named. The disease was subsequently reported in the USA in the 1914's, and soon after in almost all countries worldwide [11,12]. Every commercial poultry production is presumed to be infected, however, clinical disease is not always apparent in flocks [11]. In Colombia, there have been reports of MD since 1996 (Table 1), but it is well acknowledged that the infection was present before the 70's. The establishment of control measures by the ICA (Instituto Colombiano Agropecuario, Colombian Agricultural Institute) to prevent and control MD in poultry by resolution 573 of 1973, alerted to the problems facing the poultry industry [13].
Table 1 Colombia / Marek’s disease. Multiannual animal disease status. OIE. 2016.
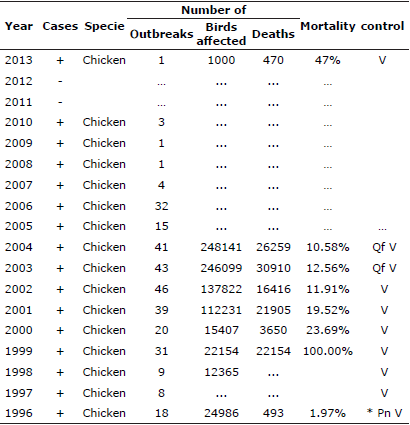
Qf. Precautions at the border; V. Vaccination; Pn. Control program for the whole country; … No information.
The evolution of MDV suggests the appearance of very pathogenic strains that may be circulating in Colombia. The classification of MDV is based on virulence factors and serotype. There are three serotypes: serotype 1 (Gallid Herpesvirus 2, GaHV-2) comprises pathogenic strains and attenuated variants; serotype 2 (Gallid Herpesvirus 3. GaHV-3) is a group of very low pathogenicity, and serotype 3 (Meleagrid Herpesvirus 1. MeHV-1) is the Herpesvirus of turkeys (HVT), which has been highly efficacious as a vaccine against MD because it is non pathogenic in chickens. There is also an additional classification for the serotype 1 that divides pathotypes based on their virulence: Mild GaHV-2 (mMDV), virulent GaHV-2 (vMDV), very virulent GaHV-2 (vvMDV) and very virulent plus (vv+MDV). Because of the different presentations of MD, there is an additional classification based on the clinical presentation: visceral form (tumors in the viscera), ocular (lymphoid infiltration in the iris), neural (paralytic disease or neuropathy) and cutaneous (tumors in skin) [3,5,14,15]. However, the last cases reported in Colombia and caused by GaHV-2 did not develop the classic presentation of the disease, and instead showed high mortality and lymphoid infiltration in peripheral nerves [16].
The GaHV-2 strains that were found included pathotypes of mMDV, vMDV and vv+MDV, suggesting combined circulation of vaccine virus and very virulent forms of the field virus to which the vaccine may not generate adequate protection, and that may lead to immunosuppresion, high mortality and lower production (personal observations). However, these undocumented cases only addressed aspects of viral spread and serotypes present, and the real pathological implications and associated production loses were undetermined.
Worldwide, MDV has become more virulent during the last decades, and unless new strategies of control are implemented, it is likely that future shifts in-virulence will occur with new variants of the currently circulating strains [9,17]. Until recently, the Colombian MDV strains were only classified based on clinical signs, histopathology results, and the phylogenetic analyses of the MEQ protein of MDV isolated in the outbreaks. In recent studies, the phylogenetic tree showed that the isolated strains clustered with the very virulent forms of GaHVvv reported worldwide [18,19]. However, confirmation that the strains belonged to the vv+ pathotype would have required doing in vivo assays in SPF chickens [15]. Such assays have not been performed in Colombia due to the lack of production of SPF eggs and the lack of isolation units to maintain SPF chickens. This situation has led to the absence of information on the circulating MDV pathotypes causing disease in the country.
More recently, the identification of MDV serotypes has been performed using conventional PCR and qPCR [17], demonstrating that all three serotypes were present in 4 layer farms and 10 backyard farms examined in Antioquia, Colombia. This information suggests that more than one strain of MDV are currently circulating in the farms which could ultimately lead to the emergence of new virulent strains. The Rispens strain vaccine (available in Colombia) against MDV have shown to allow vv+MDV strains to replicate and be shed into the environment through the vaccinated host, potentially allowing for the evolution towards increased virulence [20].
The evolution of MDV has been closely followed in the USA and Europe. There are several, yet unconfirmed, hypotheses as to why MDV evolves. The intensification of the poultry industry (high densities) and the introduction of different generations of vaccines has been suggested as the main cause of evolution.
The use of CVI988 (Rispens strain) has been continuous since 1972, with no apparent vaccine breaches until 1990. This strain is the only valid alternative against vv+MDV, so it is a real concern to the poultry industry if the MDV continues its evolution and overcomes the protection conferred by the vaccine [20,21]. It is uncertain whether MDV will continue its evolution towards greater virulence [3,8,9]. The HVT and bivalent vaccines have provided good protection for at least 10 years, so it is likely that the efficacy against these vaccines is decreasing [8,9]. Although MD is under control in almost all the world, sporadic outbreaks periodically occur in some countries with vv+ strains, suggesting that the protection with the current vaccines may be failing [8]. These highly virulent strains induce high mortality associated with brain edema in non-vaccinated chickens. In addition, they can induce lymphoproliferative lesions in the brain that are associated with neurological signs [8].
MDV: Infection, Transmission and Immunosupression. All chickens are susceptible to MDV infection. Transmission occurs via the respiratory tract, following inhalation of infectious cell-free MDV in the environment. Phagocytic activity in the lungs by innate immune system cells (macrophages and dendritic cells) results in the uptake of the virus and dissemination to lymphoid organs (bursa, thymus and spleen). The receptor for MDV on the cell surface has not been identified, therefore the molecular mechanism behind cell entry [8] at the respiratory interface is not clear. Two to three days post infection (dpi), MDV is present in the lymphoid organs, whereby infection of adaptive immune system cells is established due to its specific tropism towards B lymphocytes.
Cytolytic infection is particularly evident 5-6 dpi. The necrosis of B cell results in recruitment of inflammatory cells to the site of infection, like macrophages, T and B cells [22]. Activated T cells are a key point for the progression of pathogenesis since resting and naive T cells are refractory to MDV. These cells support viral replication and also provide the mechanism to support viral latency [23,24]. About 7 - 8 dpi the infection turns latent, and there is no evidence of lytic infection in lymphoid organs. At this point, application of histological diagnosis would provide a false negative result and PCR techniques would be required to detect MDV in lymphoid organs and peripheral blood lymphocytes [5,8].
Although MDV can infect a range of different organs within its host, the keratinized layer of stratified squamous epithelium of feather follicle is the only known location where mature virions are produced. Thus, feather follicles play a central role in the horizontal viral transmission and epidemiology of MD [5,25,26]. Infective viral particles are shed into the environment, completing the viral replication cycle, and acting as the main source for infection to naive birds through inhalation of dust or dander (Figure 3). To date, there has been no report of vertical transmission of MDV.
Like other viruses, control of MDV infection requires the coordinated responses of both the innate and adaptive immune response. The host-pathogen interaction results in a complex response with the production of soluble factors such as cytokines, antibodies, that support the function of immune system cells including macrophages, natural killer cells, T helper cells, and cytotoxic T lymphocytes [27]. Because the virus is strictly lymphoid cell-associated, T-cell mediated immune response plays an important role in the control of the infection, while the antibodies are just effective when the virus is in a free form or when the MDV antigens are expressed in the cells [28,29]. For more details, the immune response against MDV has been recently reviewed [27,30].
Diagnostic tools and current situation in Colombia. The OIE recommends different techniques to diagnose MD. Indirect techniques include ELISA, AGID, and IFI. Direct techniques include histopathology, immunohistochemistry, PCR, virus isolation and immunoprecipitation. More recently new techniques have been applied for the detection of MDV, such as qPCR and LAMP which are more sensitive, specific, reliable and faster [31,32,33]. Using the later techniques, we were able to distinguish between vvMDV-1 and the non-pathogenic MDV-1 CVI-988 vaccine in which the 132 bp repeats in the DNA were increased up to 9 repeats. Serological techniques are not fully recommended due to their poor sensitivity in an outbreak of MD. In addition, they are not useful to monitor vaccination status because antibodies do not control the infection. Consequently, direct techniques should be used instead of serological tests for adequate diagnosis and control the MDV.
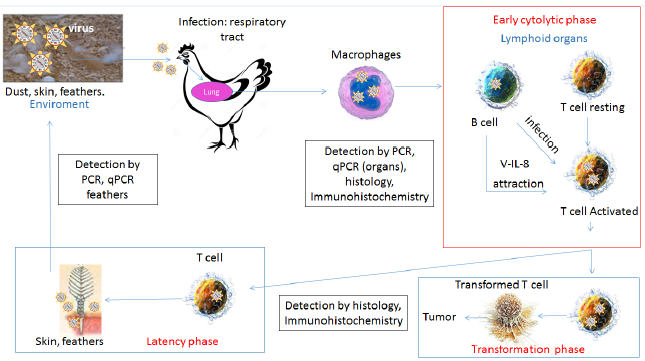
Figure 3 Viral entry into the respiratory system of young birds marks the initial infection phase. Subsequently, replication of viral particles induces pro-inflammatory signals that attract macrophages with phagocytic activity in the lungs. Infected cells migrate to lymphoid organs (spleen, thymus, bursa) and infect activated B and T lymphocytes causing the primary lytic infection of the immune system cells (early cytolitic phase). Infected T lymphocytes enter the latency phase (viral escape from immune detection). Thereafter, the virus migrates to the skin, and replicates in the feather follicle assembling the infective particles that will be released in dust and dander. In red: phase of infection. Secondary viral replication in T lymphocytes generates another round of immunosuppression and eventually tumors (transformation phase). In black square: detection method for diagnosis. Adapted from [52].
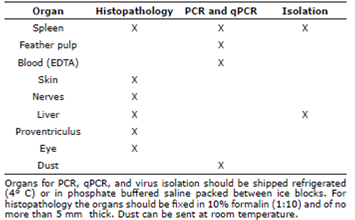
In order to identify the specific serotypes present on broiler and layer poultry farms and for monitoring vaccination status, molecular tools have been developed which allow rapid quantification of the number of the MDV genome copies in feathers and dust (qPCR) [19,34,35] . The techniques enable quantitation of Marek's disease virus genomes as copy number per million host cells. The duplex PCR measured the virus meq gene and host ovotransferrin gene in a single reaction enabling correction for differences in amount of sample DNA added. Dust can be collected from feeders and water troughs into 15 mL tubes for subsequent transport to the lab in refrigerated condition; feathers should be taken from live birds and ideally in early stages of growth (Figure 4). The qPCR in feathers provides specific information with regards to viral spread, and can be used as a tool to monitor the efficacy of the vaccination program [20,26,33,35].
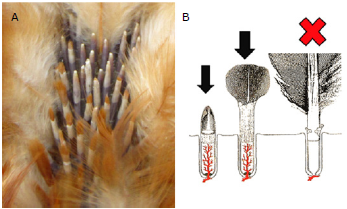
Figure 4 A. Photograph of growing feathers on a wing. B. Diagram depicting the different stages involved in feather growth. Growing feathers have a rich supply of nutrients facilitated by presence of capillaries. Fully developed feathers with resting follicle cells lack this system. Black arrow: the correct stage of the feather for the sampling. The best site to take the sample is under the wings (axillar region). Avoid the wing and tail feathers because it is painful to the birds.
It is recommended to check for the MDV presence in feathers because cell-free infectious particles are released from the feather follicle epithelium 2 to 4 weeks after infection (ie., the productive phases of MDV infection) and continues to be shed intermittently throughout the life of the chicken [36]. However, it has been reported that MDV can be detected in feathers as early as 2 dpi [3].
A qPCR method for MDV GaHV-2 is now available in Colombia (University of Antioquia, Faculty of Agrarian Sciences), and can be performed in any type of biological specimen submitted, but ideally in growing feather follicles. The normal dynamics of MDV in blood and feather from a chicken vaccinated with CVI988 at 1-day old is shown in some articles [36,39]. However, this dynamic may differ for other serotypes, or coinfection with vv+MDV [37,38]. Accurate differential measurement of the CVI988 vaccine and virulent viruses is important to investigate mechanisms of vaccinal protection. Minimal sequence differences between CVI988 and virulent MDV strains restrict the application of molecular diagnostic methods such as real-time PCR to distinguish between these viruses. Table 2 provides a list of the type of diagnostic techniques available and the ideal biological sample that should be submitted for testing.
The isolation and sequence analysis of MDV is essential for monitoring changes in MDV field strains and for the evaluation of vaccine effectiveness. In Colombia, there are reports of outbreaks of disease in layer flocks that had been vaccinated with HVT and CVI988, suggesting the immunity generated with the vaccine was not protective against the circulating field strains [16].
Determining the actual virulence of an MDV strain is not clearly defined. The USDA Avian Diseases and Oncology Laboratory (USDA-ADOL) provided the MDV pathotyping scheme based on the PIs provided by the herpes virus of turkeys (HVT) and bivalent HVT/MDV serotype 2 (MDV2) vaccines. This method has been used since 1989 and is considered the gold standard. The protocol for pathotyping requires specific chicken strains, 9 weeks of experimental period, special isolation units for infected and uninfected chickens and a high biosecurity level. Few countries can perform the protocol due to all the requirements and costs involved [39,40]. This protocol was later modified to make it easier for adoption [15,41]. Many countries began using these new protocols, but the procedures differed in the strain of viruses and chicken lines used [42]. Alternative and less costly methods have been also developed with pros and cons to the gold standard [15,43,44].
Marek disease virus in Colombia: diagnostic and control programs. To make any cases official, it is mandatory to send organs (spleen, liver, sciatic nerve, tumors) to the ICA laboratories, where techniques approved by the OIE are available, and the results are entered in the World Animal Health Information Database. This epidemiological report case will be found in the OIE B list of diseases for the country. However, techniques to further characterize any concerned strains are not performed, and these would be crucial to establish any control and preventive measures.
Currently, there are molecular techniques such as PCR and qPCR available at the University of Antioquia, which can detect viral genetic material, and can also estimate the number of genome copies present in the sample [37,38,45] which is not easily distinguishable, antigenically or genetically, from virulent Marek\u2019s disease herpesvirus. Accurate differential measurement of the CVI988 vaccine and virulent viruses is important to investigate mechanisms of vaccinal protection. Minimal sequence differences between CVI988 and virulent MDV
strains restrict the application of molecular diagnostic methods such as real-time PCR to distinguish between these viruses. Immunohistochemistry (IHC) in the affected organs can be used to associate the presence of the virus with the type of lesions. Antibodies against pp38 or Meq protein can be used in the IHC for the detection of the lytic or transformation phase of the virus [14,46,47].
Following an outbreak, the recommendation is to disinfect all the pen houses with an antiviral solution and when the chickens finish their cycle, leave the infected pen house empty for at least 4 weeks after thorough cleaning with a disinfectant solution. The farms should reinforce the biosecurity level, and always ask for verification of proper vaccination guidelines at the hatchery. In addition, it is recommended to check for viral levels (vaccine and field strains) in feathers and dust to monitor the amount of virus in the environment.
To control disease in the face of an outbreak, some authors recommend vaccination of the affected flock with CVI988. This has been shown to reduce the mortality and infection in healthy chickens [37,38,39,48,49]. However, since there is no treatment for MD, it has to be managed symptomatically and it is likely that most chickens affected with tumors or nervous signs will die sooner or later [3].
In 1992, the ICA published the national vaccination program (Resolution 811, 1992), which established that all chickens should be vaccinated at one day of age against MDV. Currently, MDV vaccination is regulated by the ICA resolutions: 3649, 3651 and 3652 of 2014, which set the requirements for the sanitary registration of poultry farms and their biosecurity certification, and also establishes a mandatory vaccination in the hatchery either at day 18 of incubation (in ovo) or day 1 of age (subcutaneous). The MDV vaccine is supposed to establish a persistent infection and good immunity that can protect against neuropathy, tumor formation and mortality. However, because the vaccines do not provide sterilizing immunity [8,50], chickens can be infected with the vaccine and field virulent strains of MDV at the same time.
In Colombia, the epidemiological situation of MD is unknown. Wild strains of MDV are believed to be present on every farm, because of increasing reports of failures in production, in both broilers and layers, associated with cases of immunosuppression. In 2014, Lopez-Osorio et al [51], studied the lymphoid organs by histopathology in growing layers from four farms in Antioquia, and found moderate and severe immunosuppression in birds under two months of age that were likely associated with the presence of immunosuppressive viruses, including MDV and CIAV (Chicken Infectious Anemia Virus).
The aforementioned authors also performed PCR and qPCR in feathers and blood to check viral infection and potential shedding, and also performed sequencing of the Meq gene. It was found that different pathotype strains were circulating in the farms (vaccine and wild mMDV and vMDV field strains) and that the birds had unusual patterns of the virus dynamics characterized by peaks of feather shedding at 30 and 60 days of age. More recently, a case of MD occurred in adult vaccinated layers with a highly pathogenic strain (vv+MDV) in the Andean region, it was characterized by high mortality rate (30%) and severe lymphoid infíltrate of the nerves (16)g. Some of the MDV cases reported by ICA between 2002 and 2012 were later confirmed by immunohistochemistry. In 2017, Lopera reported that 30.4% (17/56) of the samples in some of those cases were positives for the pp38 protein, and had no neoplastic changes, indicating that the chickens were in the cytolytic phase of the disease. [53].
The isolation and sequence analysis of MDVs in Colombia was reported in layer flocks that had been vaccinated with HVT or CVI988 [16]. However, the pathogenicity of MDVs circulating in Colombia remains unclear. The isolation and culture of MDV field strains is essential for monitoring changes in MDV field strains and for the evaluation of the vaccine effectiveness. It is essential to have an isolation unit to support rearing of SPF animals for research and to perform in vivo assay to pathotype and to isolate circulating strains, not only for MDV, but also for other chicken viruses.
In conclusion, although there is some information about the MDV strains circulating in Colombia, the dynamics of virus and the pathotypes present in the field is insufficient. Because of the lack of information regarding the pathotypes present in Colombia and that control programs are simply based on commercial criteria, current practices could even favor the appearance of more virulent forms of the virus and increase the susceptibility to other pathogens whether viral, bacterial or protozoan. It is necessary to develop techniques that allow the determination of viral pathotypes and perform routine monitoring of vaccine efficacy. With this information, veterinarians will be capable of formulating sanitary programs tailored to each specific poultry operation, and establish more rigorous vaccination program against MDV and other viral pathogens.











 texto em
texto em