Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268On-line version ISSN 1909-0544
Rev.MVZ Cordoba vol.24 no.2 Córdoba May/Aug. 2019
https://doi.org/10.21897/rmvz.1648
Research article
Describing the planktonic and bacterial communities associated with bocachico Prochilodus magdalenae fish culture with biofloc technology
1Universidad de Córdoba, Facultad de Medicina Veterinária y Zootecnia, Departamento de Ciencias Acuícolas, Instituto de Investiqación Piscícola-CINPIC, Montería, Colombia.
Objective.
To describe the planktonic communities and bacteria associated with the bocachico Prochilodus magdalenae fish culture with biofloc technology (BFT).
Materials and methods.
Bocachico fingerlings, with an average weight of 1.6±0.2 g, were stocked at three densities, i.e., 5 (T1), 10 (T2) and 20 (T3) fish/m3, with BFT in nine rectangular, 6.0 m3 concrete tanks for 120 days of culture. Identification and quantification of the microorganisms was performed every eight days in a sample of 250 ml of water per tank by analyzing aliquots on a Sedgwick-Rafter and/or in Neubauer chambers on a microscope at 10x and 40x magnification. On days 15, 45, and 90 of the fish culture, the bacterial communities were characterized by taking 2 g samples of floc and adding them to 90 ml of sterile saline solution, then subjecting them to conventional microbiological tests.
Results.
Five planktonic groups (microalgae, rotifers, cladocerans, copepods, and protists with ciliates predominating) with more rotifers and protists in the fish cultures at lower density (T1 and T2) were identified, and the largest amount of microorganisms oscillated between 174.9±21.4 ind/ml (T1) and 125.6±16.1 ind/ml (T2). It was possible to identify ten bacterial strains: Escherichia coli, Enterobacter sp., Klebsiella sp., Salmonella sp. (Enterobacteriaceae), Bacillus subtilis, Bacillus sp., Lactobacillus sp., Pseudomonas sp. (Vibrionaceae), Micrococcus sp., and Staphylococcus sp. (Coccus Gram+).
Conclusions.
The composition of plankton was similar in all treatments, with rotifers and protists being the most abundant; the bacteria showed a higher proportion of enterobacteria and heterotrophs.
Keywords: Bacteria; ciliates; rotifers; zooplankton (Source: UNESCO; CAB)
Objetivo.
Describir las comunidades planctónicas y bacterianas asociadas al cultivo de bocachico Prochilodus magdalenae con tecnología biofloc (BFT).
Materiales y métodos.
En nueve tanques rectangulares de concreto con volumen útil de 6.0 m3, se sembraron alevinos de bocachico con peso promedio de 1.6±0.2 g, a tres densidades 5 (T1), 10 (T2) y 20 (T3) peces/m3 con BFT, durante 120 días de cultivo. La identificación y cuantificación de los microorganismos se realizó cada ocho días, en una muestra de 250 ml de agua por tanque, mediante análisis de alícuotas en cámaras Sedgwick-Rafter y/o Neubauer bajo microscopio a 10x y 40x. Los días 15, 45 y 90 del cultivo se caracterizaron las comunidades bacterianas tomando una muestra de 2 g de floc en 90 ml de solución salina estéril y sometidas a pruebas microbiológicas convencionales.
Resultados.
Se identificarem cinco grupos planctónicos (microalgas, rotíferos, cladóceros, copépodos y protistas con predominancia de ciliados) con mayor cantidad de rotíferos y protistas en los cultivos con menor densidad (T1 y T2); y la mayor afluencia de microorganismos osciló entre 174.9±21.4 ind/ml (T1) y 125.6±16.1 ind/ml (T2). En el grupo de bacterias fue posible identificar 10 cepas: Escherichia coli, Enterobacter sp., Klebsiella sp., Salmonella sp. (Enterobacteriaceae) Bacillus subtilis, Bacillus sp, Lactobacillus sp, Pseduodomonas sp (Vibrionaceae), Micrococcus sp, Staphylococcus sp (Cocos gram+).
Conclusiones.
La composición del plancton fue similar en todos los tratamientos, con rotífero y protistas como los más abundantes; la mayor proporción de bacterias fueron Enterobacterias y Heterotróficas.
Palabras clave: Bacterias; ciliados; rotíferos; zooplancton (Fuente: UNESCO; CAB)
INTRODUCTION
Biofloc technology (BFT) is based on the manipulation of a microbial community under controlled conditions in a culture system, producing aquatic animals in a sustainable and biosecure way 1. This technology relies on the oxidation-reduction ratios of the nitrogen cycle and the addition of carbon-rich substrates (molasses, cassava flour, and glycerol, among others) for the sequestration and recycling of nitrogen in the form of a microbial protein 2.
These conditions stimulate the development of a beneficial microbial community, established in small flocs of organic matter re-suspended continuously in the water column by the action of permanent aeration, which recycles the ammonium nitrogen produced by the fish in the synthesis of unicellular protein. This protein can be ingested and assimilated by fish, who graze on the communities of planktonic microorganisms associated with the flocs, enabling the use of low-protein diets compared to conventional fish farming systems 3,4,5.
The transformation of particulate organic matter and other organisms in the microbial trophic chain has been proposed as a possible source of food for fish cultures produced with BFT 5. This transformation is based on the complex interactions occurring in the entire water column among the organic matter, the physical substrate, and a wide range of microorganisms. The microorganisms include microalgae, free and adhered bacteria, aggregates of organic matter, and herbivores, such as rotifers, ciliates, flagellated protozoa, and copepods 6.
As a result of these interactions, the continuous recycling of nitrogen compounds is established in the system, making available a rich, natural source of protein and lipids for the cultivated species in situ 24 hours a day 5,7,8. The assimilation of this source of food will depend mostly on the feeding habit of the species, the conditions of the system, and the populations of microorganisms that predominate. In the case of omnivorous species, such as tilapia 5,9 and shrimp, this technology is widely applicable for their cultivation 8,10,11, but such wide applicability does not exist for detritivorous species, such as bocachico Prochilodus magdalenae.
Considering the importance of natural productivity in the recycling of nutrients and maintenance of water quality, knowledge of the microbiological composition of the communities adhering to the floc is necessary in order to provide adequate management and maximize the beneficial effects for the communities involved, such as the removal of nitrogenous compounds and the feeding of farmed fishes 12. Nevertheless, little information is available about the community structure of the microorganisms present in biofloc systems, the role played by the microbial biomass, and the applicability of this technology to native fish species of commercial importance.
Therefore, since the implementation of environmentally sustainable and economically profitable intensive systems of fish production is needed, the present work characterizes the planktonic communities present in the intensive culture of bocachico in the pre-growing phase at three stocking densities using biofloc technology.
MATERIALS AND METHODS
Localization. The study was carried out at the Fish Culture Research Institute of the University of Córdoba (CINPIC, Montería, Córdoba, Colombia). A total of 630 bocachico fingerlings with 1.6±0.5 g average weight were stocked in nine rectangular, 6.0 m3 concrete tanks at three stocking densities-5 (T1), 10 (T2), and 20 (T3) fish/m3-to characterize the planktonic communities and microbiota associated with the culture of these bocachico with BFT in the pre-growing phase during 120 days of cultivation. The culture was maintained with permanent aeration supplied by a 1.5-HP blower and polyethylene diffuser tubing, and the tanks were covered with polyester mesh to reduce the light pass (60%) and to protect the fingerlings from predators.
Initial inoculum floc. The biofloc system was established with water from the bottom of a fish farming pond containing autotrophic and heterotrophic bacteria. For the promotion and development of the latter, molasses was added as a carbon source, doing so maintained the C:N ratio at nearly to 20:1. The molasses was applied using the following equation: Molasses = NT*(20-12), where 20 is the theoretical relation of C:N (20:1) for the BFT culture management, 12 is the relation of C:N (12:1) for the food rations containing 24% crude protein (2), and TN corresponds to the total amount of nitrogen in the culture, estimated via the Kubitza equation (13), where TN = [(amount of N-NH3 + N-NH4+ + amount of N-NO2 -) * volume of water] / 1000.
After the initial inoculum stabilization (14 days), based on the nitrogen compound management and the qualitative and quantitative characterization of the microorganisms present in the inoculum, 600 L of inoculum was transferred into each experimental unit, and the volume was filled to 6.0 m3 with CINPIC distribution water.
The water quality water was evaluated during stabilization and culture periods. Dissolved oxygen (DO), pH, and temperature were measured twice a day with an oximeter (YSI, 550A, USA) and a pH meter (YSI, pH100, USA). The total ammonium, nitrites, and nitrates were measured every two days, and the total hardness and total alkalinity were assessed every eight days with a photometer (YSI, 9500, USA).
Characterization of planktonic communities. Weekly, five 50-ml floc samples were taken at five different points of each culture tank and homogenized in a 250 ml Erlenmeyer flask, then 1 ml subsamples in triplicate were fixed with 10% buffered formaldehyde for the subsequent identification and quantification of the microorganisms associated with the flocs. The samples were analyzed using a Sedgwick-Rafter and/or Neubauer cameras, an optical microscope (Carl Zeiss, Axiostar, Germany), and a positive-phase-contrast inverted microscope (Carl Zeiss, Primo Vert, Germany) with objectives between 10x and 40x, and an image analyzer (Carl Zeiss, Axiovision 4, Germany). The identification of species in the different groups of plankton was carried out with the taxonomic keys of Aladro-Lubel et al 14, among others.
The abundance of microorganisms (A) per identified item was estimated with the equation: A=((Vcf)(Ni))/ (Vti)Vc, where Vcf corresponds to the final volume of the concentration, Ni is the number of counted individuals, Vti is the initial total volume, and Vc is the volume of the sample analyzed. The abundance was expressed as the number of individuals/ml (Ind/ml). The relation between the number of species and their relative abundance in time and space was analyzed weekly by treatment. Also, three ecological indices were estimated: the Shannon-Wiener index (H') for species diversity, the Pielou index (J') for uniformity, and the Simpson index (λ) for organism dominance.
Bacterial isolation and characterization. The bacterial community analyses were carried out according to the Monroy-Dosta et al. method 15. From the formation of the small flocs in the initial inoculum, and at 15, 45, and 90 days of culture, 2 g samples were taken and inoculated in 90 ml of sterile saline, with successive dilutions (1:10), and 0.1 ml was seeded in triplicate in Man-Rogosa Sharpe (MRS), Heart-Brain Infusion (HBI), Thiosulfate-Citrate-Bile Salts (TCBS), and Trypticase-soy (TSA) agar boxes. The plates were incubated at 27°C for 24h, and then the colony-forming units (CFU/ml) were counted; subsequently, through successive reseeding, the strains were purified. Gram staining was used to observe the cell morphology via microscopic observations (Carl Zeiss, Axiostar, Germany). Conventional microbiological tests were performed on isolated strains for identification of catalase, oxidase, fermentation oxide, mobility, and indoles. Finally, the identification of the strains was confirmed with the API20E, API20NE, APICHL, and APICHL50 tests. In addition to the water column bacterial samples, at the end of the culture, using a mesh of 100 urn, a sample of mud was taken from the bottom of each experimental tank and analyzed.
Statistical analysis. A randomized experimental design was utilized. The variables were checked for normality (Shapiro-Wilk test) and homoscedasticity (Bartlett's test), and then analysis of variance (ANOVA) was applied, followed by Duncan's multiple range test. In all cases, a confidence level of 95% was used. All statistical analyses were performed with SAS for Windows 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
The water quality during bocachico culture in the biofloc system is shown in Table 1. Dissolved oxygen presented averages above 6.0 mg/l; the temperature ranged between 28.6±0.3°C (T1) and 28.4±0.3°C (T3); the pH remained at 8.0, on average, in all three treatments; and the hardness ranged between 101.9±67.8 mg/l CaCO3 (T2) and 90.2 ± 52.4 mg/l CaCO3 (T1). No significant difference was observed (p>0.05) for any of these variables between the treatments. The alkalinity was significantly higher (p<0.05) in the treatments with higher densities (T2 = 114.5±57.9 mg/l CaCO3, T3=101.9±67.8 mg/l CaCO3). The highest averages of TAN (5.1±5.3 mg/l) and NH3 (2.3±2.4 mg/l) were recorded in T3, and these averages were significantly different from those for the other treatments (p<0.05). The values of NO2 - maintained an average of 0.7 mg/L in all the treatments; the average values of NO3 - were significantly higher (p<0.05) in the treatments with higher densities (T2 = 19.2±12.3 mg/l, T3 = 19.2±2.2 mg/l) (p>0.05).
Table 1 Parameters of water quality during the cultivation of P. magdalenae in the pre-growing phase with biofloc technology.
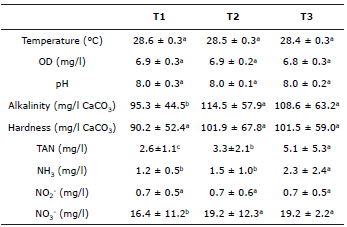
Different letters in the same row indicate a significant difference (p<0.05).
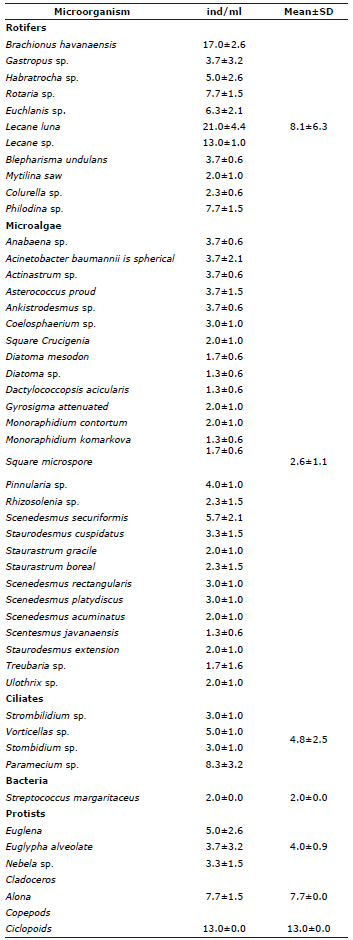
Planktonic microorganisms present during the stabilization and maturation of the floc inoculum. The microorganism taxa identified during the stabilization and maturation period of the floc inoculum are listed in Table 2. The copepods were the most abundant (13.0±0.0 ind/ml), showing a significant difference in abundance (p<0.05) from the other groups. Rotifers and microalgae were the groups with the greatest diversity of species. In the rotifers, the species Lecane luna (21.0±4.4 ind/ml) and Brachionus havanaensis (17±2.6 ind/ml) were the most abundant, while, for the microalgae, Scenedesmus securiformis (5.7±2.1 ind/ml) and Pinnularia sp. (4.0±1.0 ind/ml) predominated.
Table 2 Microorganisms identified during the stabilization and maturation of the floc inoculum prior to the start of the cultivation of P. magdalenae in the pre-growing phase with biofloc technology.
Characterization of planktonic microorganisms at the different densities of bocachico culture. The zooplankton groups identified during the culture period in the pre-growing phase utilizing biofloc technology are shown in Table 3. The greatest abundance of zooplankton was registered in T1 (174.9±21.4 ind/ml) and the lowest in T3 (121.3±15.9 ind/ml), with a significant difference (p<0.05). In the lower stocking density tank (T1), a greater abundance of rotifers (35.6±18.6 ind/ml), protists (69.6±28.6 ind/ml), annelids (14.3±4.2 ind/ml), amoebas (11.4±2.3 ind/ml), and cladocerans (6.6±2.2 ind/ml) was observed, with these groups showing significant differences between stocking densities (p<0.05). In T1 (18.8±10.2 ind/ml) and T3 (13.9±12.1 ind/ml), the highest abundance of ciliates was recorded without significative differences (p>0.05). The group of copepods showed no significant difference between treatments (p>0.05), ranging from 5.2±1.5 ind/ml (T3) and 6.7±1.5 ind/ml (T1).
Table 3 Abundance of zooplankton in the cultivation of P. magdalenae with biofloc technology.
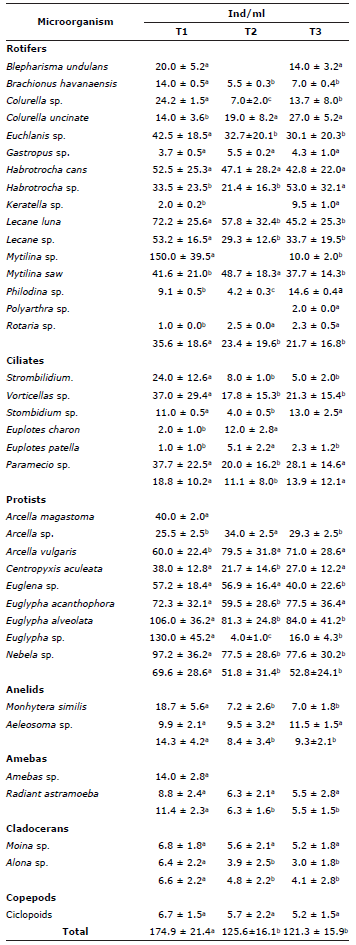
Different letters in the same row indicate a significant difference (p<0.05).
Among the rotifers, Euchlanis sp., Habrotrocha lata, Habrotrocha sp., Lecane luna, Lecane sp., Mytilina sp., and Mytilina videns were the most abundant; among the ciliates, the morphospecies that were the most abundant were Vorticellas sp. and Paramecio sp. The most abundant protists were Arcella vulgaris, Euglypha acanthophora, Euglypha alveolata, Euglypha sp., and Nebela sp.
Table 4 shows the abundance of the species and morphospecies of phytoplankton identified. The most abundant were Ankistrodesmus acicularis (31.5±8.0 cells/ml, T1) (p<0.05), Ankistrodesmus sp. (25.5±6.4 cells/ml, T3) (p<0.05), Crucigenia quadrata (50.5±2.0 cells/ml, T1 and T2) (p>0.05), Monoraphidium komarkova (50.9 ±22.3 cells/ml, T3) (p<0.05), and Scenedesmus securiformis (30.2±10.2 cells/ml, T1) (p<0.05).
Table 4 Abundance of phytoplankton species (cells/ml) identified during the cultivation of P. magdalenae with biofloc technology (Mean ± SD).

Different letters in the same row indicate a significant difference (p<0.05).
Ecological indices. The ecological indices evaluated in the planktonic communities during cultivation are described in Table 5. The Shannon-Wiener index registered the highest value in T1 (2.5-3.2 bits/ml) and T2 (2.4-3.2 bits/ml), which were significantly different that found for T3 (1.8-2.8 bits/ml) (p<0.05), except for weeks 9, 10, 14, and 16 since no significant difference (p>0.05) was found between these weeks. The Pielou uniformity index was higher in T1 (0.8-0.9) and T2 (0.8-0.7) during the first two weeks of culture; the lowest values of this index (0.6-0.7) were recorded in T1 in weeks 9, 10, and 15; no significant difference was observed for the remaining weeks among the treatments. Except for the 6th and 12th week of culture, the dominance (Simpson index) did not present a statistic difference between the treatments, ranging between 0.7 and 0.9 (p>0.05).
Table 5 Ecological indices of the planktonic microorganisms associated with the cultivation of P. magdalenae with biofloc technology. Different letters in the same row indicate a significant difference (P<0.05) between treatments for each week of culture.
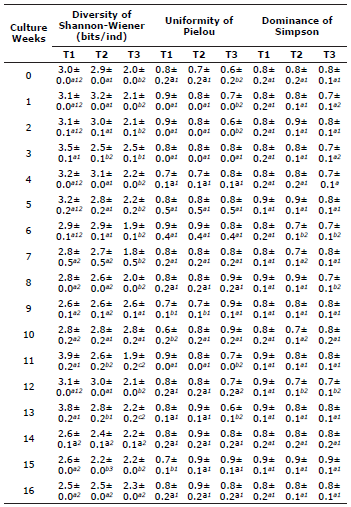
Different numbers in the same column indicate a significant difference within each treatment (p<0.05).
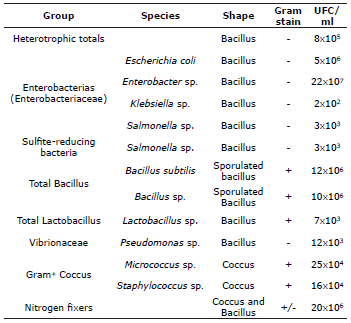
Bacterial characterization. In the initial inoculum, eight groups were identified (Table 6) and included bacterial colonies from the family Enterobacteriaceae, such as colonies of Escherichia coli, Enterobacter sp., Klebsiella sp., and Salmonella sp., which are characterized as gram-negative bacilli and occurred in concentrations of 2.0x102 to 22.0x107 CFU/ml. A group of sporulated Gram+ bacilli, i.e., Bacillus subtili, and Bacillus sp., were identified; these bacilli are related to the species Lactobacillus sp. Bacteria functionally described as heterotrophic, sulfite-reducing, cocci, and nitrogen-fixing were also identified.
Table 6 Bacterial groups isolated in the period of stabilization and maturation of the floc inoculum used in the culture of P. magdalenae with biofloc technology.
The qualitative characterization of the bacterial groups identified (group/species), relative to their presence/ absence during the four months of the culture of P. magdalenae with biofloc technology are listed in Table 7. In addition to the groups present initially in the inoculum, other colonies of bacteria were also present, such as Bacillus cereus, Vibrio cholerae, and Vibrio sp. The groups of bacterial observed in the mud at the bottom of the culture were similar to those identified previously, and heterotrophic bacteria was prevalent.
Table 7 Bacterial groups identified in the different treatments evaluated in the cultivation of P. magdalenae in the pre-growing phase with biofloc technology. +: presence, -: absence.
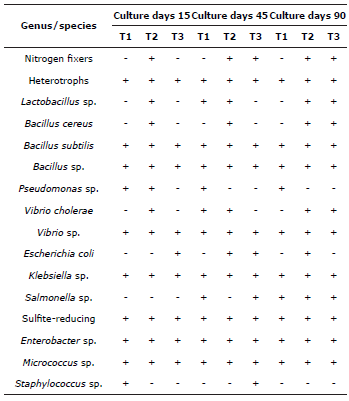
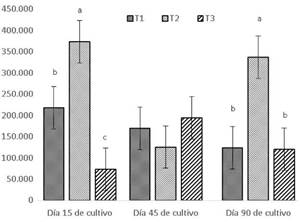
Figure 1 shows the CFU/ml of the groups of bacteria identified at 15, 45, and 90 days of culture. In T2, the highest CFU/ml were present on day 15 (373.000 CFU/ ml) and day 90 (336.000 CFU/ml), whereas the lowest dominance was recorded in T3 on day 15 (73.667 CFU/ ml) and day 90 (120.000 CFU/ml), with a significant difference between these treatments (p<0.05).
DISCUSSION
In the present study, the parameters of water quality (OD, temperature, and pH) did not show differences between treatments; these parameters are considered optimal for the development of culture in biofloc systems 6,8,16 and are appropriate for the management of bocachico 17; also, the alkalinity and hardness are considered adequate for the maintenance and the nitrification route of the bacteria in the system 9,4,12.
The values of TAN and NH3 were higher at the highest density evaluated (20 fish/m3), which seems to be a consequence of the greater amount of food offered, along with the higher proportion of organic matter present in this treatment. In tilapia cultures with BFT, lower values were reported: between 1.9 and 2.5 mg/l of TAN 13. This result suggests that TAN values should be stabilized by bacterial activity or by the joint action between the planktonic communities and the microbiota, where the toxic fraction of these compounds is assimilated and incorporated for efficient recycling of the nutrients. NH3 is a compound that is toxic to fish, even at very low concentrations; concentrations above 1.5 mg/l in commercial-scale cultures are considered lethal 2,8,18, with acceptable levels being below 0.5 mg/l 19. In the present study, the average levels of NH3 were similar to those reported by several authors, who evaluated the behavior and dynamics of the biofloc system in the performance of fish and shrimp cultures, mostly with values not higher than 3.0 mg/l 9,20,21.
In BFT culture, high levels of nitrogen compounds (TAN, NH3) are present; but the toxicity of these compounds will depend on the management conditions and the degree of tolerance of the cultivated species. Since, although they were well above the optimum values for the management of the system 2,20,21, these levels did not affect the system's management and the welfare of the species in culture, these findings suggest the tolerance of the species to the registered concentrations of these nitrogenous compounds. No reports of a medium lethal dose (LC50) were found in the literature for the bocachico; therefore, it is necessary to study the tolerance of this species to these nitrogen compounds.
The nitrite values remained similar in all treatments, whereas the nitrates at the higher stocking densities (T2 and T3) were higher. This dynamic can be attributed to the management offered as a function of the analyzed density, i.e., the food ration and therefore the organic matter load were greater in T3. In general, the water quality of the culture is associated with the management conditions of the system, with some parameters being affected; in particular, the nitrogen compounds were affected by the evaluated densities. Importantly, the alimentary habit of the bocachico (iliophagous-detritivorous) 22 is a factor to be considered when considering the adaptation of this cultivation system for species with this feeding habit.
The composition of the plant communities associated with the culture of bocachico in BFT suggests a culture system rich in microorganisms of biological and nutritional importance, with nutrient flow and availability of primary food. Six planktonic groups were identified (not including bacteria) in association with the macro-aggregates of floc, such as microalgae, rotifers, ciliates, cladocerans, protists, and copepods, with a greater abundance of the latter group being observed in the stabilization period of the inoculum, whereas there was a greater abundance of rotifers, protists, and ciliates during the pre-growing phase. These results agree with the reports of other authors that have characterized the planktonic communities of macro-aggregates during the conformation and stabilization of the inoculum, as well as in the development of cultures with this technology 8,12,15.
The abundance of microorganism groups was related to the stocking density; therefore, some pressure from the species on the abundance of the microorganisms present can be inferred, depending on the density of fish evaluated and the time of stabilization of the system. This pressure was described by Ballester et al 23 and Miaca et al 24, who suggested that the elements that produce the floccules, such as the carbon source and balanced feed as well as the fish in the system, can have a direct influence on the groups of developed organisms and their abundance to the cultivation time.
In the present study, the resuspension of solids in the water column was observed. This resuspension is suggested to be a consequence of the behavior and feeding habit of the bocachico (benthic-iliophagous-detritus), a phenomenon that has been observed in fish with similar behaviors 25, i.e., causing strong agitation on the bottom, increasing the turbidity, and facilitating the flow of nutrients through the trophic chain 26. Although excessive resuspension could affect primary productivity, possibly, at 20 fish/m3 (T3), excessive pressure from grazing will be felt in the generation and reproductive cycles of the microorganisms.
In the culture with the BFT systems, the interaction and dominance of the planktonic and bacterial communities are presented as a significant contribution in the protein and energy requirements for the species of farming interest. This contribution is made through the flow of nutrients through the established trophic chains from primary producers to secondary consumers, preferably through the consumption of microalgae and bacteria through continuous grazing in macroaggregates of floc 12,27.
The protists were the group with the highest density (Table 3). Protists are an important group in the elimination of contaminants, especially nitrogen compounds, contributing to the formation of bioaggregates and flocs 28; in the depredation of bacterial populations that can become pathogenic 29; and in the formation, distribution, and composition of the bacterial community 28.
The rotifers groups constitute another of the representative groups within the trophic chains present in the culture environments with BFT 12. In the present study, rotifers were the group with the second greatest density, which oscillated in the reported range (5 to 196 ind/ml) of a similar study 12,15. Associated in the same way with the dynamics of the system and with the formation of the macroaggregates, the ciliates (Vorticellas sp. and Paramecium sp.) were also characterized in the tilapia and shrimp cultures developed in the biofloc system 15,24,27.
When considering the less abundant species, the presence of Monhytera similis is noteworthy within the group of annelids, a group considered to be of great importance within the biofloc system due to its high content of crude protein and essential fatty acids; as well as species of the amoebas, the cladocerans, and the copepods. The genera Ankistrodesmus and Scenedesmus (Chlorophyta) were predominant in the microalgae, sometimes referenced for the quantity and quality of their nutrients and for the selective consumption of certain species of ciliates and rotifers that control microalgal populations 12.
The characterization of the groups above as microorganisms is recurrent in most studies of culture with BFT 12,15,16,23,27. Therefore, the establishment of a specific, dominant, uniform and diverse planktonic community is suggested, with characteristics such as reproductive strategy, small size, short life cycle, and broad tolerance to environmental factors. These microorganisms can also become established due to system conditions, such as carbon source, initial inoculum, and, as observed in the present study, according to the behavior and nutritional habits of the cultivated species.
The diversity of the microorganisms found showed a maximum value of 3.9 bits/ind, with high dominance (>0.7) and uniformity (>0.6). When identified by group, the rotifers and microalgae were the groups with the highest taxonomic richness and abundance during cultivation. These results allow us to infer that an index of low diversity may indicate that few species exist relative to the total of some individuals characterized in BFT systems. In general, the BFT system is not a closed production system, and, when selected inoculum are used, the pattern of microorganisms and planktonic communities associated with macroaggregates of floc is predictable.
The microbiota present affect the nutrient dynamics in the system; these were characterized for Enterobacteriaceae, Bacillus, Lactobacillus, heterotrophic, sulfite-reducing, Coccus, and nitrogen-fixing bacteria, agreeing with the reports of Monroy-Dosta et al. 15. Those authors when examining the microbial community associated with the biofloc in a tilapia culture, determined that heterotrophic bacteria, such as Pseudomonas, Bacillus, Vibrios, Enterobacter, and Micrococcus, were present.
This type of bacteria promotes defined routes in the elimination of toxic nitrogen compounds in farming systems. Accordingly, Ebeling et al. 4 defined the elimination of nitrogen by photoautotrophic algae, its immobilization by heterotrophic bacteria of the microbial protein biomass, and its chemo-autotrophic oxidation in nitrate by nitrifying bacteria as the main routes within the flow of nutrients in BFT culture systems. The relative importance of each one varies according to the type and intensity of the production system. In the present study, although immobilization of the heterotrophic bacteria was encouraged, the system was dominated by nitrifying bacteria.
Microbial communities in BFT systems are presented in a variety of ways and include opportunistic bacteria and pathogens as well as neutral and beneficial bacteria. The presence of colonies of the genera Bacillus and Lactobacillus, genera with probiotic characteristics in the development of fish culture, is emphasized mainly because these genera secrete a great variety of exoenzymes and polymers that generate a hostile environment for pathogenic bacteria 30. The maintenance of the system by the established bacterial communities is notable, although routes of nitrification and recycling of nitrogen compounds exist under the minimal conditions of nutrients and the management of water quality in suitable ranges for the cultivation of the species.
The results of the present study allow us to conclude that the composition of the planktonic and bacterial communities was similar in all the treatments, with the rotifer, protists, and ciliates groups being the most abundant, all sources of food for bocachico. The present microbiota is directly related to the dynamics of the system, with a higher quantity of enterobacteria and heterotrophic bacteria.
REFERENCES
1. Vinatea L, Gálvez A, Browdy C, Stokes A, Venero J, Haveman J, Lewis B, Lawson A, Shuler A, Leffler G. Photosynthesis, water respiration and growth performance of Litopenaeus vannamei in a super-intensive raceway culture with zero water exchange: interaction of water quality variables. Aquacultural Engineering 2010; 42(1):17-24. DOI: https://doi.org/10.1016/j.aquaeng.2009.09.001 [ Links ]
2. Avnimelech Y. Biofloc Technology - A Practical Guidebook. The World Aquaculture Society; 2009. https://books.google.com.co/books/about/Biofloc_Technology.html?id=FI-CrgEACAAJ&redir_esc=y [ Links ]
3. Wasielsky J, Atwood H, Stokes A, Browdy C. Effect of natural production in a zero exchange suspended microbial floc based super-intensive culture system for white shrimp Litopenaeus vannamei. Aquaculture 2006; 258:396-403. DOI: https://doi.org/10.1016/j.aquaculture.2006.04.030 [ Links ]
4. Ebeling J, Timmons M, Bisogni J. Review of autotrophic and heterotrophic bacterial control of ammonia-nitrogen in zero-exchange production systems: stoichiometry and experimental verification. Aquaculture 2006; 257:346-358. DOI: https://doi.org/10.1016/j.aquaculture.2006.03.019 [ Links ]
5. Avnimelech Y. Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture 2007; 264:140-147. DOI: https://doi.org/10.1016/j.aquaculture.2006.11.025 [ Links ]
6. Ray A, Lewis B, Browdy C, Leffler J. Suspended solids removal to improve shrimp Litopenaeus vannamei, production and an evaluation of a plant based feed in minimal-exchange, superintensive culture systems. Aquaculture 2010a; 299:89-98. DOI: https://doi.org/10.1016/j.aquaculture.2009.11.021 [ Links ]
7. Ekasari J, Crab R, Verstraete W. Primary nutritional content of Bio-flocs cultured with different organic carbon sources and salinity. HAYATI Journal of Biosciences. 2010; 17(3):125-130. DOI: https://doi.org/10.4308/hjb.17.3.125 [ Links ]
8. Crab R, Defoirdt T, Bossier P, Verstraete W. Biofloc technology in aquaculture: Beneficial effects and future challenges. Aquaculture 2012; (356-357):351-356. https://doi.org/10.1016/j.aquaculture.2012.04.046 [ Links ]
9. Azim M, Littlea D. The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia Oreochromis niloticus. Aquaculture 2008; 283(1-4):29-35. https://doi.org/10.1016/j.aquaculture.2008.06.036 [ Links ]
10. Kunh D, Lawrence A, Boardman G, Patnaik S, Marsh L, Flick G. Evaluation of two types of bioflocs derived from biological treatment of fish effluent as feed ingredients for Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2010; 303(1-4):28-33. https://doi.org/10.1016/j.aquaculture.2010.03.001 [ Links ]
11. Suita S, Ballester E, Abreu P, Wasielesky WJr. Dextrose as carbon source in the culture of Litopenaeus vannamei (Boone, 1931) in a zero exchange system. Latin American Journal of Aquatic Research 2015; 43(3): 526-533. http://www.lajar.cl/pdf/imar/v43n3/Articulo_43_3_13.pdf [ Links ]
12. Ray A, Seaborn G, Leffler J, Wilde S, Lawson A, Browdy C. Characterization of microbial communities in minimal-exchange, intensive aquaculture systems and the effects of suspended solids management. Aquaculture 2010b; 310:130-138. DOI: https://doi.org/10.1016/j.aquaculture.2010.10.019 [ Links ]
13. Kubitza F. Criacão de tilapia em sistema com bioflocos sem renovacão de água. Panorama da Aquicultura 2011; 14-23. URL Disponible en http://www.acquaimagem.com.br/docs/Pan125_Kub_bioflocos_piscicultura.pdf . [ Links ]
14. Aladro-Lubel M. Manual de protozoarios [Manual of protozoa]. Faculty of Sciences, Autonomous University of Mexico, Mexico 2009; p 123 [ Links ]
15. Monroy-Dosta M, De Lara-Andrade R, Castro-Mejia J, Castro-Mejia G, Coelho-Emerenciano M. Composición y abundancia de comunidades microbianas asociadas al biofloc en un cultivo de tilapia. Rev Biol Mar Oceanogr. 2013; 48(3):511-520. http://dx.doi.org/10.4067/S0718-19572013000300009 [ Links ]
16. Emerenciano M, Gaxiola G, Cuzon G. Biofloc Technology (BFT): A Review for Aquaculture. Application and Animal Food Industry. INTECH 2013; 12:301-327. DOI: http://dx.doi.org/10.5772/53902 [ Links ]
17. Garcia JJ, Celis LM, Villalba EL, Mendoza LC, Brú SB, Atencio VJ, Pardo SC. Evaluation of the bocachico polyculture Prochilodus magdalenae and Oreochromis niloticus tilapia using perifiton fixing surfaces. Rev Fac Med Vet Zoot. 2011; 58(2):71-83. https://revistas.unal.edu.co/index.php/remevez/article/view/25398 [ Links ]
18. Hargreaves J. Biofloc Production Systems for Aquaculture. SRAC 2013; 4503:8-10. https://aquaculture.ca.uky.edu/sites/aquaculture.ca.uky.edu/files/srac_4503_biofloc_production_systems_for_aquaculture.pdf [ Links ]
19. Pérez-Fuentes J, Hernández-Vergara M, Pérez-Rostro C, Fogel I. C:N ratio affect nitrogen removal and production of nile tilapia Oreochromis niloticus raised in a biofloc system under high density cultivation. Aquaculture 2016; 452:247-251. https://doi.org/10.1016/j.aquaculture.2015.11.010 [ Links ]
20. Schveitzer R, Arantes R, Costódio P, Espírito-Santo C, Arana LV, Seiffert W, Andreatta E. Effect of different biofloc levels on microbial activity, water quality and performance of Litopenaeus vannamei in a tank system operated with no water exchange. Aquacultural Engineering 2013; 56:59-70. https://doi.org/10.1016/j.aquaeng.2013.04.006 [ Links ]
21. Lorenzo M, Souza E, Schledera D, Rezendea P, Seifferta W, Vieiraa F. Intensive hatchery performance of Pacific white shrimp in the biofloc system under three different fertilization levels. Aquacultural Engineering 2016; 72-73:40-44. https://doi.org/10.1016/j.aquaeng.2016.04.001 [ Links ]
22. Marrugo-Negrete J, Navarro-Frómeta A, Ruiz-Guzman J. Total mercury concentrations in fish from Urrá reservoir (Sinú river, Colombia). Six years of monitoring. Rev MVZ Córdoba 2015; 20(3): 4754 4765. https://doi.org/10.21897/rmvz.45 [ Links ]
23. Ballester E, Abreu P, Cavalli R, Emerenciano M, Abreu L, Wasielesky W. Effect of practical diets with different protein levels on the performance of Farfantepenaeus paulensis juveniles nursed in a zero exchange suspended microbial flocs intensive system. Aquaculture Nutrition 2010; 16: 163-172. https://doi.org/10.1111/j.1365-2095.2009.00648.x [ Links ]
24. Maicá P, Borba M, Wasielesky W. Effect of low salinity on microbial floc composition and performance of Litopenaeus vannamei (Boone) juveniles reared in a zero water-exchange super-intensive system. Aquaculture Research 2012; 43:361-370. https://doi.org/10.1111/j.1365-2109.2011.02838.x [ Links ]
25. Della Rosa P, Ortiz J, Cáceres A, Sánchez S, Roux J. Desempeno del sábalo Prochilodus lineatus en policultivo con pacú Piaractus mesopotamicus. Lat Am J Aquat Res 2016; 44(2):336-341. http://dx.doi.org/10.3856/vol44-issue2-fulltext-14. [ Links ]
26. Milstein A, Ahmed AF, Masud OA, Kadir A, Wahab MA. Effects of the filter feeder silver carp and the bottom feeder's mrigal and common carp on small indigenous fish species (SIS) and pond ecology. Aquaculture 2006; 258 (1-4): 439-451. https://doi.org/10.1016/j.aquaculture.2006.04.045 [ Links ]
27. Loureiro K, Wilson W, Abreu P. Use of protozoa, rotifers and nematodes as live feed for shrimp cultured in the BFT system. Atlântica Rio Grande2012; 34(1):5-12. https://doi.org/10.5088/atl.2012.34.1.5 [ Links ]
28. Abreu C, Ballester E, Odebrecht C, Wasielesky WJr, Cavalli R, Granéli W, Anésio A. Importance of biofilm as food source for shrimp (Farfantepenaeus paulensis) evaluated by stable isotopes. J Exp Mar Bio Ecol. 2007; 347 (1-2):88-96. https://doi.org/10.1016/j.jembe.2007.03.012 [ Links ]
29. Wu L, Peng C, Peng Y, Li L, Wang S, Ma Y Effect of wastewater COD/N ratio on aerobic nitrifying sludge granulation and microbial population shift. Journal Environment Science. 2012; 24(2):234-241. https://doi.org/10.1016/S1001-0742(11)60719-5 [ Links ]
30. Monroy D, Castro B, Fernández P, Mayorga R. Inhibition of Aeromonas hydrophila by probiotic strains isolated from the digestive tract of Pterophyllum scalare. Rev Mex Ing Quím 2010; 9(1):37-42. http://rmiq.org/iqfvp/Pdfs/Vol9%20 no%201/RMIQ_Vol9_No_1_5.pdf [ Links ]
How to cite (Vancouver) Ayazo-Genes J, Pertúz-Buelvas V, Jiménez-Velásquez C, Espinosa-Araujo J, Atencio-García V, Prieto-Guevara M. Describing the planktonic and bacterial communities associated with bocachico Prochilodus magdalenae fish culture with biofloc technology. Rev MVZ Cordoba. 2019; 24(2):7209-7217. DOI: https://doi.org/10.21897/rmvz.1648
Creative Commons Attribution 4.0 International License This article is distributed under the terms of the (https://creativecommons.org/licenses/by-sa/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source. 
Received: July 01, 2018; Accepted: November 01, 2018; Published: May 01, 2019











 text in
text in