Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268On-line version ISSN 1909-0544
Rev.MVZ Cordoba vol.24 no.3 Córdoba Sep./Dec. 2019 Epub June 01, 2020
https://doi.org/10.21897/rmvz.1412
Research article
Use of disaccharides and activated carbon to preserve cellulolytic ruminal bacterial consortiums lyophilized
1 Universidad Autónoma de Guerrero, Facultad de Medicina Veterinaria y Zootecnia No. 2. Carretera Acapulco-Pinotepa Nacional Kilómetro 197, Cuajinicuilapa, Guerreo, México, C.P. 41940.
2 Colegio de Postgraduados, Programa de Ganadería. Carretera México-Texcoco Km 36.5, Montecillos, Edo. De México, México, C.P. 56230.
Objective.
To determine in vitro fermentation of cellulolytic ruminal bacterial consortia (CBC) preserved by lyophilization using activated carbon, maltose and lactose as preservatives.
Materials and methods.
A CBC was isolated from the ruminal fluid of a female water buffalo in selective cellulolytic media. The CBC were lyophilized without preservative (SP), activated carbon (CA), lactose (LA) o maltose (MA) as preservatives. The experimental design was completely random to measure biogas at different time intervals; as well as completely random with 4x3 factorial arrangement, factors were preservative [SP, CA, LA and MA] and fermentation time (24, 48 and 72 h) for pH, ammoniacal nitrogen (NH3-N), dry matter degradation (DMD), neutral detergent fiber degradation (NDFD), enzymatic activity cellulases and total bacteria population.
Results.
LA produced higher accumulated biogas at 72 h and partial biogas after 12 h (p≤0.05). SP did not show differences (p>0.05) in cellulases, total bacteria population, DMD and NDFD in the fermentation times evaluated with the rest of the preservative.
Conclusions.
The production of partial and accumulated biogas, the increase in the degradation rate of 8.3 and 91.1% in the DMD and NDFD from 24 to 72 h (p≤0.05) in the LA preservative, show that lactose can be used as a preservative of ruminal cellulolytic bacteria.
Keywords: In vitro fermentation; lactose; lyophilization maltose; preservatives (Source: DeCS)
Objetivo.
Determinar la fermentación in vitro de consorcios bacterianos ruminales celulolíticos (CBC) conservados por liofilización usando carbón activado, maltosa y lactosa como preservadores.
Materiales y métodos.
Un CBC se aisló de fluido ruminal de una búfala de agua en medios selectivos celulolíticos. Los CBC se liofilizaron con carbón activado (CA), lactosa (LA) o maltosa (MA) como preservadores y sin preservador (SP). El diseño experimental fue completamente al azar para medir biogás a diferentes intervalos de tiempo; así como, un diseño completamente al azar con arreglo factorial 4x3, los factores fueron preservadores (SP, CA, LA y MA) y tiempo de fermentación (24, 48 y 72 h) para pH, nitrógeno amoniacal (N-NH3), degradación de materia seca (DMS) y de fibra detergente neutro (DFDN), actividad enzimática celulasas y la población de bacterias totales.
Resultados.
LA produjo mayor biogás acumulado a las 72 h y parcial a partir de las 12 h (p≤0.05). SP no mostró diferencias (p>0.05) en celulasas, conteo de bacterias total, DMS y DFDN en los tiempos de fermentación evaluados con el resto de los preservadores.
Conclusiones.
La producción de biogás parcial y acumulada, el aumento en la tasa de degradación de 8.3 y 91.1 % en la DMS y DFDN de las 24 a 72 h (p≤0.05) con el preservador LA, muestran que la lactosa puede usarse como preservador de bacterias celulolíticas ruminales.
Palabras clave: Fermentación in vitro; lactosa; liofilización; maltosa; preservadores (Fuente: DeCS)
INTRODUCTION
Microorganisms are fundamental in biogeochemical cycles. Their preservation is vital in microbiology laboratories because of their biotechnological potential. Microbial resources need to be kept in good physiological and genetically stable condition 1. The main conservation methods are continuous growth, dehydration and freezing. Lyophilization is widely used to conserve and transport biological products, and is important in scientific research 2. However, protein denaturation and DNA damage may occur during the process due to osmotic shock and membrane injury, consequently decreasing cell viability 3. Preservatives are required to mitigate the osmotic pressure and stress induced by frostbite and dehydration because individual microorganisms and even strains of a given species vary in their viability after lyophilization 2. Only limited information is available on the use of preservatives during lyophilization of ruminal microorganisms 4.
There are three general categories of preservatives. One kind penetrates both the cell wall and the cytoplasmic membrane to make the membrane more plastic and prevent formation of ice crystals inside the cell during freezing. Another penetrates only the cell wall to induce cell plasmolysis prior to freezing. And a third does not interact directly with the cell wall or cytoplasmic membrane at all, but forms a viscous layer that inhibits the ice formation rate by increasing solution viscosity 5.
As preservatives, sugars act on cell membranes by inhibiting phase changes detrimental to low hydration, and reducing gel transition temperatures to the fluid phase 6. Disaccharides are used as preservatives because they stabilize lipid membranes due to the temperature difference between the vitreous transition and formation of the sugar in a vitreous state 2. For example, lactose (1 to 10%) has been used as a preservative in Lactococcus lactis, providing better protection than with glycerin in cultures stored at -70 °C 7. Maltose in combination with glycerol has been used in the preservation of Scenedesmus spp.
Activated charcoal exhibits characteristics of reversible physical adsorption, and adsorption in liquid phase without elimination by simple desorption and porosity, allowing its use as a microorganism preservative 4. When used to preserve a cellulolytic bacteria consortium it reduced crystalline cellulose degradation compared to a preservative without activated carbon 4.
Studies involving microorganism conservation using preservatives are generally based on preservative viability at rehydration, while others measure preservative effect during microorganism fermentation 4,8,9,10. The hypothesis of the present study is that lactose, maltose and activated charcoal can function as preservatives of cellulolytic bacteria consortia without affecting their potential growth during in vitro fermentation. The study objective was therefore to evaluate in vitro fermentation of cellulolytic ruminal bacteria consortia conserved by lyophilization and using activated charcoal, maltose and lactose as preservatives.
MATERIALS AND METHODS
Study site. The study was carried out in the Laboratory of Animal Nutrition of the Faculty of Veterinary Medicine and Animal Husbandry No. 2 of the Autonomous University of Guerrero, located in the municipality of Cuajinicuilapa, in the state of Guerrero, Mexico (16°08” N; 98°23” W). At an altitude of 50 masl, climate is mostly warm sub- humid with summer rains, an average annual precipitation of 1200 mm and an annual average temperature of 25°C 11.
Ethical concerns. The cellulolytic bacteria consortia were obtained from ruminal fluid extracted from a female water buffalo. The donor animal was handled following the internal bioethics and welfare regulations of the Autonomous University of Guerrero and the federal regulation addressing animal experimentation (NOM-062-ZOO-1999) 12.
Culture medium. The medium contained 30 mL clarified ruminal fluid [i.e. fresh bovine ruminal fluid centrifuged for 10 min at 12,857 x g and sterilized (All American® 1941X, USA) for 15 min at 121 °C and 15 psi]; 5 mL mineral solution I [6 g K2HPO4 (J.T. Baker®) in 1000 mL distilled water]; 5 mL mineral solution II [6 g KH2PO4 (J.T. Baker®) + 6 g (NH4)2SO4 (J.T. Baker®) + 12 g NaCl (Meyer®) + 2.45 g MgSO4 (Meyer®) + 1.6 g CaCl-2H2O (Meyer®) in 1000 mL distilled water]; 0.1 mL 0.1% resazurine (Sigma-Aldrich®); 0.2 g soy peptone (MCD Lab®); 0.1 g yeast extract (BD Bioxon®); 2 mL cysteine-sulfide solution [2.5 g L-cysteine (Sigma-Aldrich®) in 15 mL 2N NaOH (Meyer®) + 2.5 g Na2S-9H2O (Meyer®) buffered in 100 mL distilled water]; 5 mL 8% Na2CO3 solution (J.T. Baker®) and 52.6 mL distilled water. The medium was sterilized for 15 min in an autoclave at 121°C and 15 psi 4.
Cellulolytic bacteria consortium. Ruminal fluid was extracted from a water buffalo (Bubalus bubalis) cow using an esophageal probe. Before sampling, the cow (500 Kg LW) had been grazing in a pangola grass (Digitaria decumbes) pasture at 56 days regrowth, without feed supplements. The fluid was centrifuged for 3 min at 1.157 x g (Metrix Velocity 14, USA), and the supernatant recovered for use as an inoculum. Under a biosafety hood (Labconco®, USA) at low CO2 flow, 9 mL sterile culture medium were added to test tubes (Pirex®, Mexico; 18 x 150 mm) containing 0.05 g mulatto grass (Brachiaria hybrid cv. CIAT 36087) at 65 d of sterile regrowth, and kept for 24 h at 39°C in an incubator (Ecoshel 9082, Mexico) to verify sterility. For each tube, 1 mL inoculum was added in triplicate and incubated for 72 h at 39°C. One milliliter of this inoculated medium was transferred to another tube containing sterile medium and grass, and incubated for 72 h at 39°C. Five transfers were done to obtain the cellulolytic bacteria consortium (CBC).
Preservatives. Culture medium (27 mL), cellobiose (0.1% of medium; Sigma-Aldrich®) and sterile carboxymethylcellulose (0.1% of medium; Meyer®) were placed in serological vials (60 mL). These were left to rest under constant CO2 flow for 24 h at 39°C to confirm sterility. The vials were inoculated with 3 mL of the product resulting from the fifth transfer and incubated for 72 h at 39°C. Four preservatives were tested: 1) NP, no preservative, as a control; 2) AC, activated charcoal, 1 mL 30% AC solution (Hycel®) (p/v; 30 g 100 mL-1 distilled water); 3) LA, lactose, 1 mL 30% lactose solution (Meyer®) (p/v; 30 g 100 mL-1 distilled water); and 4) MA, maltose, 1 mL 30% maltose solution (Meyer®) (p/v; 30 g 100 mL-1 distilled water). The vials were inclined at 25° to extend the contact surface during lyophilization, initially frozen to -38°C, and then lyophilized at -49°C and 0420 mBar for 24 h (Labconco®, Freezone 6 L, USA).
CBC reactivation. Under CO2 flow, 9 mL culture medium, and sterile cellobiose (0.1%) and carboxymethylcellulose (0.1%) were added to sterile test tubes (18 x 150 mm) and incubated for 24 h to confirm sterility. Ten tubes were inoculated with 0.05 g of lyophilized NP, AC, LA or MA, and incubated under CO2 flow for 72 h at 39°C. Using sterile serological (120 mL) vials, 45 mL culture medium, and sterile cellobiose (0.1%) and carboxymethylcellulose (0.1%) were incubated under CO2 flow for 24 h to confirm sterility. These were inoculated with 5 mL of one of the reactivated CBC (NP, AC, LA or MA) and incubated for 48 h at 39°C to produce an inoculum for use in the in vitro gas production test.
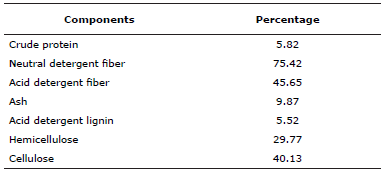
In vitro gas production. Substrate was mulatto grass harvested at 63 d regrowth and dehydrated at 60°C to constant weight in a stove (Felisa® FE-293A, Mexico). Particle size was reduced in a Thomas-Wiley mill (Thomas Scientific®, Swedesboro, NJ, USA) with a 1 mm sieve. Substrate crude protein (CP), ash (As) and organic matter (OM) contents were quantified using AOAC techniques (13). Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were measured with the ANKOM Technology Method, according to Van Soest et al (14) (Table 1).
The biodigesters were serological glass vials (120 mL) containing 0.5 g mulatto grass and 45 mL culture medium. The biodigesters were kept under anaerobic conditions with CO2, hermetically sealed with a neoprene plug (20 mm Ø) and an aluminum ring. The biodigesters were then sterilized for 15 min at 121°C and 15 psi, and incubated for 24 h at 39°C to confirm sterility 15. After sterilization they were inoculated with NP, LA, MA or AC (10 independent replicates) and incubated for 72 h at 39°C. Biogas production was measured at 6, 12, 24, 48 and 72 h incubation 16, based on displacement of the plunger in a glass syringe (50 mL; BD Yale®, Brazil). In addition, at 24, 48 and 72 h incubation, measurements were taken of pH, total bacteria, ammoniacal nitrogen, cellulase activity, dry matter and neutral detergent fiber degradation.
pH. Medium pH was measured with a potentiometer (Hanna® HI2211, Italy; calibration: pH 7 and 4).
Total bacteria count. Medium (1 mL) from the middle portion of the biodigester was mixed with 0.25 mL formaldehyde (Sigma-Aldrich®) at 10% in a test tube. Total bacteria count was done by direct counting in a Petroff-Hausser chamber 4,15.
Ammoniacal nitrogen (NH3-N). Medium (1 mL) from the biodigester was mixed with 0.25 mL metaphosphoric acid (Meyer®) at 25% (4:1 ratio), the mixture centrifuged for 25 min at 3,500 x g and the supernatant recovered in 2 mL vials. Supernatant (20 µL) was mixed with 1 mL phenol solution [10 mg Na2 [Fe(CN)5NO]*2H2O (Meyer®) + 10 g phenol crystals (Meyer®) completed to 1000 mL with distilled water] and 1 mL hypochlorite solution [7.5 g NaOH (Reasol®) + 21.3 g Na2HPO4 (Meyer®) + 15 mL hypochlorite (5%; Reasol®), completed to 1000 mL with distilled water]. The mixture was incubated for 30 min at 37°C in a water bath, 5 mL distilled water added to dilute it, and it was then mixed with a vortex agitator (Genie 2 G-560, USA). Absorbance was measured at 630 nm in a UV-VIS spectrophotometer (Jenway® 6850, USA) calibrated with an ammoniacal nitrogen concentration method (r2 = 0.9994) 17.
Cellulase enzymatic activity. This was measured with the reducing sugars concentration method 18. Culture medium (2 mL) was centrifuged for 25 min at 9.710 x g and 4°C and the supernatant used as an enzymatic extract. The substrate was 0.5% carboxymethylcellulose (2.5 g carboxymethylcellulose in 500 mL 50 mM citrate buffer, at pH 4.8) (Meyer®). The standard curve was prepared with a 10 mM glucose solution [0.18 g dextrose (Merck®) 100 mL-1 50 mM citrate buffer, at pH 4.8] (r2 = 0.9994). The reaction mixture for each sample contained 1.8 mL substrate and 0.2 mL enzymatic extract. Incubation was done in test tubes, in two replicates, for 60 min at 50°C, 3 mL DNS were added, the samples boiled for 5 min and immediately cooled on ice. Absorbance was measured at 540 nm. A blank was prepared for each sample with 1.8 mL substrate. These were incubated for 60 min at 50°C, 3 mL DNS and 0.2 mL enzymatic extract added, the mixture boiled for 5 min and immediately cooled on ice. Again, absorbance was measured at 540 nm. One unit (U) was defined as the amount of enzyme released by 1 µmol min-1 glucose under the above reaction conditions.
Dry matter (DMD) and neutral detergent fiber degradation (NDFD). The sample remaining in the biodigester was filtered through filter bags (ANKOM® 541) to constant weight and moisture eliminated by drying at 60°C for 24 h in a kiln. Calculation of DMD was done with the formula DMD (%) = (initial sample - residual sample/initial sample) * 100 16. The filter bags were heat sealed and NDF content quantified 14. Degradation of NDF as a percentage (% NDFD) was calculated with the formula NDFD (%) = (initial NDF - residual NDF/initial NDF) * 100 16.
Results analysis. A completely randomized design was used to analyze biogas production at 6, 12, 24, 48 and 72 h, and accumulated production at 72 h (10 independent samples). Means were compared with the Tukey test (p≤0.05). The statistical model was Yij = μ + τi + εij; where: Yij = response variable in the i-th preservative and j-th repetition; μ = general mean; Τi = effect of the i-th preservative; and Εij = random error, normally distributed with a mean of 0 and σ2 variance.
Cellulase enzymatic activity (3 independent samples), DMD, NDFD (6 independent samples), NH3-N, bacteria count (5 independent samples) and pH (10 independent samples) were analyzed with a completely randomized design with a 4 x 3 factorial arrangement. Factors were the four tested preservatives (NP, AC, LA and MA) and three fermentation times (24, 48 and 72 h). The means were adjusted by least squares using the PROC LSMEANS in the SAS® package 19, and were compared with the adjusted Tukey test. The statistical model was Yijk = Μ + Ai + Bj + ABij + Εijk; where Yijk = response variable; Μ = general average; Ai = effect of preservative factor; Bj = effect of incubation time factor; ABij = effect of preservative and incubation time interaction; and Εijk = random error.
RESULTS
The LA preservative produced the highest (p≤0.05) accumulated biogas value at 72 H. Compared to the control (NP), biogas production was 31.9% in the LA treatment and 16.8% higher in the AC (p≤0.05). Accumulated biogas production at 72 h did not differ (p>0.05) between the MA and NP treatments (Table 2). At 6 h, the AC treatment exhibited the highest (p≤0.05) biogas production, but from 12 h on production was highest in the LA treatment (p≤0.05) (Table 2). Compared to the control (NP), biogas production in LA increased 52.3% at 48 h and 81.8% at 72 h (p≤0.05). Production in the AC treatment did not differ (p>0.05) from NP at 48 or 72 h. Finally, in the MA treatment production increased (p≤0.05) 19.4% at 48 h and 39.5% at 72 h (Table 2).
Table 2 Biogas production (mL g-1 DM) up to 72 h incubation of mulatto grass with lyophilized ruminal cellulolytic consortia with and without disaccharides and activated carbon as preservatives.
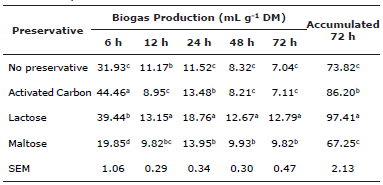
a,b,c Different letter superscripts in the same column indicate significant difference (p≤0.05). SEM = Standard error of the mean.
Because in vitro fermentation was done at different times, analyses were done of the main factors and their interactions (Table 3) to document any changes as fermentation time increased (Table 4). In the NP treatment (control) dry matter degradation (DMD) values did not differ (p>0.05) between the evaluated fermentation times (Table 4). None of the four preservative treatments (NP, LA, MA and AC) exhibited differences (p>0.05) in DMD at 72 h incubation. Likewise NDFD did not differ (p>0.05) between the three incubation times in any of the treatments (Table 4); it averaged 3.7% at 24 h, 5.0% at 48 h and 6.4% at 72 h.
Table 3 Significance (p values) for fermentation characteristics at 24, 48 and 72 h incubation of mulatto grass with lyophilized ruminal cellulolytic bacteria consortia with and without disaccharides and activated carbon as preservatives.
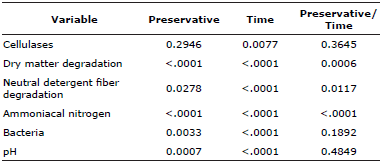
Table 4 Ammoniacal nitrogen concentration, and dry matter and neutral detergent fiber degradation at 24, 48 and 72 h incubation of mulatto grass with lyophilized ruminal cellulolytic bacteria consortia with and without disaccharides and activated carbon as preservatives&.
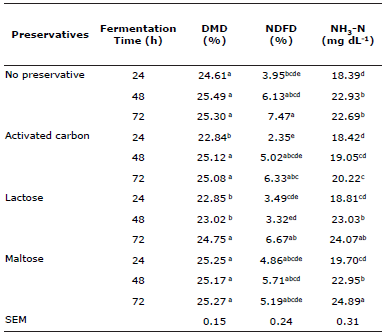
& The variables exhibited a significant preservative/incubation time interaction (p≤0.05).
a,b,c,d,e Different letter superscripts in the same column indicate significant difference (p≤0.05).
DMD = dry matter degradation; NDFD = neutral detergent fiber degradation; NH3-N = ammoniacal nitrogen; SEM = standard error of the mean.
At 24 h incubation, all treatments exhibited no differences (p>0.05) in biodigester NH3-N concentration (Table 4), averaging 18.8 mg dL-1. Concentrations also did not differ (p>0.05) between 48 and 72 h in the NP, AC and LA treatments. However, these treatments did increase (p≤0.05) NH3-N concentration between 24 h and 48 h incubation: 24.0% in NP; 6.5% in AC and 25.19% in LA. Concentration in the MA treatment increased (p≤0.05) with incubation time.
Cellulase enzymatic activity did not differ between preservatives (p>0.05), averaging 90.33 mU mL-1. Among the incubation times, this activity was 3.6% higher (P≤0.05) at 24 h than at 48 h; but at 72 h activity did not differ (p>0.05) from the other incubation times (Table 5).
Table 5 Cellulase enzymatic activity, total bacteria count and pH at 24, 48 and 72 h incubation of mulatto grass with lyophilized ruminal cellulolytic bacteria consortia with and without disaccharides and activated carbon as preservatives.&
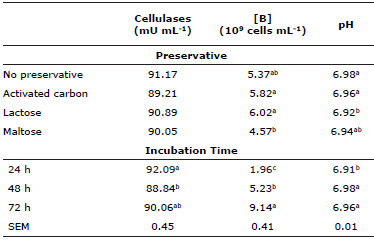
& The variables did not exhibit a significant preservative/incubation time interaction (p>0.05).
a,b,c Different letter superscripts in the same column indicate significant difference (p≤0.05).
Cellulases = cellulase enzymatic activity; [B]= total bacteria count; SEM = standard error of the mean.
Total bacteria count did not differ (p>0.05) from the control (NP) in the three preservative treatments (Table 5). However, counts did increase (p≤0.05) 166.8% between the 24 and 48 h incubation times, and 74.7% (p≤0.05) from 48 to 72 h (Table 5). Finally, pH ranged from 6.91 to 6.98, near neutral, regardless of treatment (Table 5).
DISCUSSION
Microorganism conservation is important in many research fields, and genetic consistency is crucial in the conserved bacteria 3. Partial biogas production values indicate the carbohydrate fermentation trajectory; cell content is fermented during the first 24 h and structural carbohydrates are fermented thereafter 15. For all the evaluated preservatives the present biogas production values were lower than previously reported for the same preservatives tested with a CBC from a water buffalo cow inoculated into cobra grass 15; cumulative production in this study was 103.4 mL g-1 DM. These differences in biogas production values can be attributed to variations in nutrient availability in mulatto and cobra grasses 20, microorganism efficiency in use of grass, origin of the ruminal CBC and microorganism density 21.
Differences also existed between biogas production at different times in the control (NP) in the present study and those reported previously (61.7 mL g-1 DM at 9 h, 14.3 at 12 h, 6.7 at 24 h, 11.2 at 48 h and 9.5 at 72 h) 15; DMD values also differed between the controls in the present and the previous study. In the present study the CBC were reactivated after lyophilization. This technique can damage cell wall structures, cell membranes and DNA, causing diminished cell viability after CBC reactivation 3. However, in the previous study the CBC had not been lyophilized 15. The discrepancy between this study and the present study is particularly notable in the LA treatment in which biogas production increased beginning at 24 h. This probably occurred because lactose stabilizes cell membranes due to the difference in the vitreous transition temperature 2, and inhibits adverse phase changes at low hydration 6.
None of the tested preservatives drastically improved dry matter degradation capacity (DMD). This contrasts with previous studies in which addition of preservatives is reported to protect bacteria during the freezing and drying of the lyophilization process 2,3,5,6,7,8. However, use of these preservatives has only been studied in terms of bacteria viability, without addressing their variable effects on in vitro fermentation, which did form part of the present study. Slight increases (P ≤ 0.05) in DMD were observed in the LA treatment from 24 to 72 h (8.3%), and the AC treatment between 24 and 48 h (10.1%). Even these slight increases are noteworthy since structural carbohydrate fermentation begins at 24 h 15, and in the CBC evaluated in the present study DMD improved beginning at 24 h (Table 3). Nonetheless, DMD values higher than those for the NP, LA, MA and AC treatments at 72 h have been reported in two previous studies 4,15; DMD was reported as 29.65% in cobra grass inoculated with a CBC, and 32.75% on Whatman Paper® + crystalline cellulose inoculated with a lyophilized CBC and activated charcoal as a preservative. Discrepancies between these results and the present values may be attributed to CBC origin and type, CBC microbial population profile, CBC donor species, and/or substrate nutrient composition 20.
Use of lactose, activated charcoal and maltose as preservatives during CBC lyophilization did not improve or decrease NDFD upon CBC reactivation for in vitro fermentation with mulatto grass. However, the overall NDFD rate did increase from 24 to 72 h incubation: 6.7% in MA; 89.1% in NP, 91.1% in LA, and 169.4% in AC. A study using CBC from a water buffalo cow reported a rate slightly higher than the MA treatment when using cobra grass (10.67%) but lower with corn stover (3.94%) 15. Dry matter and neutral detergent fiber degradation by CBC are attributed to the need of bacteria to interact with other microorganisms by cross-feeding 4,15,22, and their capacity for catabolic repression in the presence of glucose or other compounds in the medium that inhibit enzymatic synthesis 15,23. This has been confirmed by improved degradation rates of CBC when cultivated with ruminal bacteria 15.
Cellulolytic bacteria use ammoniacal nitrogen (NH3-N) as their sole nitrogenous source 24,25, and concentration depends on the degradability of the nitrogen fractions in the diet 26. When using only mulatto grass, soy peptone and yeast extract in the biodigesters, NH3-N levels responded to CBC behavior during the 72 h incubation period (Table 4). Use of lactose and maltose as CBC preservatives had no affect (p≤0.05) on NH3-N concentration over time. In contrast, activated charcoal lowered NH3-N concentration. Nonetheless, with all the evaluated preservatives and incubation times NH3-N concentrations (Table 4) remained within reported ranges 27. Similar results (18.26 mg dL-1) were reported in a study using cobra grass in biodigesters inoculated with water buffalo CBC 15. A lower concentration (14.7 mg dL-1) was found in the rumen of water buffaloes fed rice straw and a concentrate (0.3% of body weight) 28.
None of the preservative treatments affected cellulase enzyme production or total bacteria count in the lyophilized and reactivated CBC. Other studies have found increased activity: 5.32 U mL-1 with Clostridium thermocellum incubated at 37°C and pH 7.2 29; and 0.38 U mL-1 with Providencia sp. incubated at 37°C for 24 h 9. The low cellulase yield in the present study can be attributed to anaerobic fermentation conditions 29, since temperature and pH are the most important factors influencing cellulose hydrolysis and production 9, as well as substrate type and composition preceding the CBC 29, and incubation time in the enzymatic activity determination method 30.
The increased estimated bacteria population observed here at different incubation times was due to proliferation of cellulolytic bacteria beginning at 24 h, since these ferment structural carbohydrates 15, and that the inoculum used was a CBC. However, the growing microbial population contradicts the cellulose activity results since the cellulase concentration did not increase in response to the bacterial population. Growth in the bacterial population in the present study may also be attributed to the pH values being within the range for correct functioning, since pH values lower than 6.0 inhibit enzymatic activity 15,10. A study of cobra grass and corn stover inoculated with CBC found pH values similar to the present results but lower total bacteria counts 15.
Overall the present results suggest that lactose is an effective preservative of cellulolytic ruminal bacterial consortia. This is supported by the LA treatment’s partial and accumulated biogas production results, its increased (p≤0.05) dry matter and neutral detergent fiber degradation rates from 24 to 72 h, and the absence of changes in NH3-N content, medium pH and total bacteria count compared to the control CBC. Maltose and activated charcoal also exhibited positive results in certain variables of in vitro fermentation. Further research is needed to determine if lactose is an effective preservative under other conditions, and if maltose and activated carbon could function better as preservatives of ruminal cellulolytic bacterial consortiums.
Acknowledgements
The research reported here was financed by the Consejo Nacional de Ciencia y Tecnología via Project 253275, Ciencia Básica CB-2015-01, “Elaboración de un probiótico a partir de bacterias celulolíticas aisladas de búfalos de agua y bovinos para mejorar la degradación in vitro de los principales forrajes usados en la alimentación de rumiantes”.
REFERENCES
1. Morales-García YE, Duque E, Rodríguez-Andrade O, de la Torre J, Martínez-Contreras RD, Pérez-y-Terrón R, y Muñoz-Rojas J. Bacterias preservadas, una fuente importante de recursos biotecnológicos. Biotecnología. 2010; 14(02):11-29. https://www.researchgate.net/profile/Jesus_Munoz-Rojas/publication/235901617_Bacterias_Preservadas_una_Fuente_Importante_de_Recursos_Biotecnologicos/links/0912f513faa98c769b000000.pdf [ Links ]
2. Boge L, Västberg A, Umerska A, Bysell H, Eriksson J, Edwards K, et al. Freeze-dried and re-hydrated liquid crystalline nanoparticles stabilized with disaccharides for drug-delivery of the plectasin derivative AP114 antimicrobial peptide. J Colloid Interface Sci. 2018; 522:126-135. DOI: https://doi.org/10.1016/j.jcis.2018.03.062 [ Links ]
3. Krumnow AA, Sorokulova BI, Olsen E, Globa L, Barbaree MJ, and Vodyanoy JV. Preservation of bacteria in natural polymers. J Microbiol Methods. 2009; 78(2):189-194. DOI: https://doi.org/10.1016/j.mimet.2009.05.017 [ Links ]
4. Sánchez-Santillán P, Cobos-Peralta MA, Hernández-Sánchez D, Álvarado AI, Espinosa-Victoria D, y Herrera-Haro JG. Use of activated carbon to preserve lyophilized cellulolytic bacteria. Agrociencia 2016; 50(5):575-582. https://www.colpos.mx/agrocien/Bimestral/2016/jul-ago/art-3.pdf [ Links ]
5. Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, and Gibbs P. Effects of Various Sugars Added to Growth and Drying Media upon Thermotolerance and Survival throughout Storage of Freeze-Dried Lactobacillus delbrueckii ssp. Bulgaricus. Biotechnol. 2004; 14(10): 248−254. DOI: https://doi.org/10.1016/j.idairyj.2004.02.001 [ Links ]
6. Lenne T, Bryanta G, Garveyb CJ, Keiderlingc U, Kosterd KL. Location of sugars in multilamellar membranes at low hydration. Physica B. 2006; 385-386(2):862-864. DOI: https://doi.org/10.1016/j.physb.2006.05.127 [ Links ]
7. Hubalek Z. Protectants used in the cryopreservation of microorganisms. Cryobiology. 2003; 46(3):205-229. DOI: https://doi.org/10.1016/S0011-2240(03)00046-4 [ Links ]
8. Lu Y, Huang L, Yang T, Lv F, Lu Z. Optimization of a cryoprotective medium to increase the viability of freeze-dried Streptococcus thermophilus by response surface methodology. Food Sci Technol. 2017; 80:92-97. DOI: http://dx.doi.org/10.1016/j.lwt.2017.01.044 [ Links ]
9. Poszytek K, Ciezkowska M, Sklodowska A, Drewniak L. Microbial consortium with high cellulolytic activity (mchca) for enhanced biogas production. Front Microbiol. 2016; 7:324-334. DOI: https://doi.org/10.3389/fmicb.2016.00324 [ Links ]
10. Sánchez-Santillán P, Cobos-Peralta MA. In vitro production of volatile fatty acids by reactivated cellulolytic bacteria and total ruminal bacteria in cellulosic substrate. Agrociencia. 2016; 50(5):565-574. https://www.colpos.mx/agrocien/Bimestral/2016/jul-ago/art-2.pdf [ Links ]
11. INEGI. Anuario estadístico y geográfico de los Estados Unidos Mexicanos. Instituto Nacional de Estadística Geografía e Informática. (Acceso el 01 de octubre de 2018). URL disponible en URL disponible en www.beta.inegi.org.mx/app/areasgeograficas/?ag=12023 [ Links ]
12. NOM-062-ZOO-1999. Norma Oficial Mexicana, Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. SENASICA, México. 22 de agosto de 2001. URL disponible en https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf. [ Links ]
13. AOAC. Official Methods of Analysis (19th) Association of official Analytical Chemist. Arlington (VA), Washington DC: AOAC; 2012. http://www.aoac.org/aoac_prod_imis/AOAC_Docs/OMA/OMA19Revisions.pdf [ Links ]
14. Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991; 74(10):3583-3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2 [ Links ]
15. Herrera-Pérez J, Vélez-Regino L, Sánchez-Santillán P, Torres-Salado N, Rojas-García A, Maldonado-Peralta M. In vitro fermentation of fibrous substrates by wáter buffalo ruminal cellulolytic bacteria consortia. Rev MVZ Córdoba. 2018; 23(3):6860-6870. DOI: https://doi.org/10.21897/rmvz.1374 [ Links ]
16. Hernández-Morales J, Sánchez-Santillán P, Torres-Salado N, Herrera-Pérez J, Rojas-García AR, Reyes-Vázquez I, Mendoza-Núñez MA. Composición química y degradaciones in vitro de vainas y hojas de leguminosas arbóreas del trópico seco de México. Rev Mex Cienc Pecu. 2018; 9(1):105-120. DOI: http://dx.doi.org/10.22319/rmcp.v9i1.4332 [ Links ]
17. McCullough H. The determination of ammonia in whole blood by a direct colorimetric method. Clin Chim Acta. 1967; 17(2):297-304. https://doi.org/10.1016/0009-8981(67)90133-7 [ Links ]
18. Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Biochem. 1959; 31(3):426-428. https://pubs.acs.org/doi/abs/10.1021/ac60147a030 [ Links ]
19. SAS. Statistical Analisys Sofware, SAS/STAT. Version 9.33 Edition. Cary (NC): SAS institute Inc; 2011. [ Links ]
20. Elghandour MMY, Kholif AE, Lopez S, Mendoza GD, Odongo NE, and Salem AZM. In vitro gas, methane and carbon dioxide productions of high fibrous diet incubated with fecal inocula from horses fed live yeasts in response to the supplementation with different yeast additives. J Equine Vet Sci. 2016; 38:64-71. DOI: http://dx.doi.org/10.1016/j.jevs.2015.12.010 [ Links ]
21. Zicarelli F, Calabrò S, Cutrignelli MI, Infascelli F, Tudisco R, and Bovera F. In vitro fermentation characteristics of diets with different forage/concentrate ratios: comparison of rumen and faecal inocula. J Sci Food Agric. 2011; 91(7):1213-1221. DOI: https://doi.org/10.1002/jsfa.4302 [ Links ]
22. Duarte A, Luna RS, Starns HD, Weckerly FW. Intraspecific scaling of rumen-reticulum fill might depend on dietary fiber. Am Midl Nat. 2014; 172(2):329-337. https://doi.org/10.1674/0003-0031-172.2.329 [ Links ]
23. Thurston B, Dawson KA, Strobel HJ. Pentose utilization by the ruminal bacterium Ruminococcus albus. Appl Environ Microbiol. 1994; 60(4):1087-1092. https://www.ncbi.nlm.nih.gov/pubmed/8017905 [ Links ]
24. Russell JB, O’Connor JD, Fox DG, Van Soest PJ, Sniffen CJ. A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. J Anim Sci. 1992; 70(11):3551-3561. https://doi.org/10.2527/1992.70113551x [ Links ]
25. Carro MD, López S, Valdés C, Ranilla MJ. Effect of nitrogen supplementation on the in vitro rumen fermentation of nitrogen deficient forages. Arch Zootec. 1999; 48(183):295-306. http://www.uco.es/organiza/servicios/publica/az/php/img/web/02_03_24_05carro.pdf [ Links ]
26. Rodríguez MC, Aguirre E, Salvador F, Ruiz O, Arzola C, La OO, Villalobos C. Producción de gas, ácidos grasos volátiles y nitrógeno amoniacal in vitro con dietas basadas en pasto seco. Revista Cubana de Ciencia Agrícola. 2010; 44(3):251-259. http://www.redalyc.org/pdf/1930/193015664007.pdf [ Links ]
27. Chandrasekharaiah M, Thulasi A, Suresh KP, Sampath KT. Rumen degradable nitrogen requirements for optimum microbial protein synthesis and nutrient utilization in sheep fed on finger millet straw (Eleucine coracana) based diet. Anim Feed Sci Technol. 2011; 163(2-4):130-135. DOI: https://doi.org/10.1016/j.anifeedsci.2010.10.015 [ Links ]
28. Chanthakhoun V, Wanapat M, Kongmun P, Cherdthong A. Comparison of ruminal fermentation characteristics and microbial population in swamp buffalo and cattle. Livest Sci. 2012; 144(3):172-176. DOI: https://doi.org/10.1016/j.livsci.2011.11.011 [ Links ]
29. Otajevwo FD, Aluyi HSA. Cultural conditions necessary for optimal cellulase yield by cellulolytic bacterial organisms as they relate to residual sugars released in broth medium. Mod Appl Sci. 2011; 5(3):141-151. https://doi.org/10.5539/mas.v5n3p141 [ Links ]
30. Galindo J, Marrero Y, González N, Aldama AI. Caracterización de la actividad celulolítica en el líquido de rumen filtrado. Rev Cub Cienc Agríc. 2004; 38(3):259-263. http://www.redalyc.org/pdf/1930/193017849006.pdf [ Links ]
Como citar (Vancouver). Texta NJ, Sánchez-Santillán P, Hernández SD, Torres-Salado N, Crosby GM, Rojas-García R, et al. Uso de disacáridos y carbón activado para preservar consorcios de bacterias ruminales celulolíticas liofilizadas. Rev MVZ Cordoba. 2019; 24(3):7305-7313. DOI: https://doi.org/10.21897/rmvz.1412
Received: October 2018; Accepted: March 2019; Published: August 2019











 text in
text in