Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268On-line version ISSN 1909-0544
Rev.MVZ Cordoba vol.24 no.3 Córdoba Sep./Dec. 2019 Epub June 04, 2020
https://doi.org/10.21897/rmvz.1536
Research article
Effect of homeopathic medicines on growth, survival and gastrointestinal microbiota of juvenile scallop Argopecten ventricosus
1 Centro de Investigaciones Biológicas del Noroeste, S.C. (CIBNOR). Av. IPN 195, Col. Playa Palo de Santa Rita Sur. La Paz, B.C.S., México.
2 Universidad Central de Las Villas (UCLV-CBQ). Carretera a Camajuaní Km 5 ½. Santa Clara, Villa Clara, CP. 54830, Cuba.
3 Centro de Investigación en Materiales Avanzados (CIMAV). Chihuahua, Chih. México.
4 Universidad Técnica Estatal de Quevedo (UTEQ).Facultad de Ciencias Ambientales, Quevedo, Los Ríos EC.120501, Ecuador.
Objective.
To study the effect of homeopathic medicines on growth, survival and gastrointestinal (GIT) microbiota of Catarina scallop Argopecten ventricosus.
Materials and methods.
Five homeopathic (HOM) treatments derived from bacteria [(T1) ViP-ViA 1D, (T2) ViP-ViA 7C], minerals [(T3) AcF-MsS 1D, (T4) PhA-SiT 7C] or venoms [T5) ViT 31C] and three controls: [(C1) diluted ethanol 1:99, (C2) diluted/succussed ethanol 1C and (C3) distilled water] were evaluated (21 days) in triplicate. Microbiota was analysed by sequencing the V3-V5 region of the 16S rRNA genes.
Results.
The best growth in shell-length corresponded to T1 (117 µm d-1) and T2 (108 µm d-1) and the highest survival to T3 and T5, stating T3 as the best HOM-treatment. A clear separation was found in rarefaction curves of HOM-treated against un-treated control scallops. Significant differences (p≤0.05) were found for Phyla (Proteobacteria> Actinobacteria> Firmicutes> Bacterloidetes>Chloroflexi and for Genera: Symbiobacterium> Microbacterium> Methylobacillus> Bacillus> Paenibacillus> Burkholderia> Nostoc> Methylobacterium> Leucobacter). The genus Symbiobacterium was dominant in T5, finding significant differences (p≤0.05) with respect to all treatments. At species level, Microbacterium maritypicum (Actinobacteria) showed a greater relative abundance (p≤0.05) in T1 and T3 and Symbiobacterium toebii (Firmicutes) was also significantly higher (p≤0.05) in abundance in T5 and T2, both against initial T0.
Conclusions.
This study showed for a first time, the composition of GIT microbiota in A. ventricosus and focused on the potential applicability of homeopathy to improve overall performance and modulate the GIT microbiota of the species.
Keywords: Aquacultural homeopathy; scallop microbiota; performance; 16S rRNA
Objetivo.
Estudiar el efecto de medicamentos homeopáticos sobre el crecimiento, supervivencia y microbiota del tracto gastrointestinal (TGI) de almeja Catarina Argopecten ventricosus.
Materiales y Métodos.
Se aplicaron cinco tratamientos homeopáticos derivados de bacterias [(T1) ViP-ViA 1D, (T2) ViP-ViA 7C], minerales [(T3) AcF-MsS 1D, (T4) PhA-SiT 7C], o venenos [(T5) ViT 31C] y tres controles: (C1) etanol diluido 1:99, (C2) etanol dinamizado 1C y (C3) agua destilada. La microbiota se determinó secuenciando la región V3-V5 del gen 16S rRNA.
Resultados.
El mayor crecimiento en longitud de la concha correspondió a T1 (117 µm d-1) y T2 (108 µm d-1), la mayor supervivencia a T3 y T5 y el mejor resultado global a T3. Las curvas de rarefacción de los grupos tratados y controles mostraron una clara separación. Se encontraron diferencias significativas (p≤0.05) entre filos (Proteobacteria > Actinobacteria > Firmicutes > Bacterloidetes> Chloroflexi y para los Géneros: Symbiobacterium > Microbacterium > Methylobacillus > Bacillus > Paenibacillus > Burkholderia > Nostoc > Methylobacterium > Leucobacter). El género Symbiobacterium fue dominante (p≤0.05) para T5, respecto a todos los tratamientos y grupos controles. La especie Microbacterium maritypicum (Actinobacteria) mostró la mayor abundancia relativa (p≤0.05) en T1 y T3 y Symbiobacterium toebii (Firmicutes) en T5 y T2 (p≤0.05), ambas con respecto al inicio del estudio T0.
Conclusiones.
Se presenta por primera vez la composición de la microbiota del TGI de A. ventricosus y la aplicabilidad potencial de la homeopatía para mejorar el rendimiento productivo y modular la microbiota gastrointestinal de la especie.
Palabras clave: Homeopatía acuícola; microbiota de almeja; rendimiento; 16S rRNA
INTRODUCTION
Commercially important scallops are produced along the Pacific coast and the Gulf of California, Mexico, but scallop fishing is considered unsustainable 1. Catarina scallop Argopecten ventricosus (Sowerby II, 1842) is a highly valued and rapid growth cultivable species 2,3 but hatchery spat production 4 is affected by massive mortalities during first growth phases 5,6. To prevent/reduce disease and avoid massive larvae and spat mortalities in hatchery, antibiotics and other chemotherapeutics are traditionally used, which have different side effects on the environment 7. They induce development and spread of antibiotic resistant strains 8,9, accumulate in tissues of treated organisms 10 and affect their gastrointestinal tract (GIT) microbiota 11.
The structure and activity of GIT has effect on the host and helps to keep equilibrium with the surrounding environment. Different strategies have been developed exploiting this interaction to modulate the GIT microbial community, in order to promote growth, health and enhanced aquaculture productivity 6,11,12.
This is insufficient, so more efficacious and eco-sustainable approaches such as aquacultural homeopathy are urgently required 13-17, which are mineral, plant and animal derived products that exert their action at ultra-low doses according to the “similarity principle”, increasing host natural immunity and resistance 18. Homeopathic treatments may also reduce or substitute the use of antibiotics, hormones, disinfectants, and non-eco-friendly substances; they also decrease production costs and increase quality and innocuity of farmed molluscs, fishes and crustaceans 13-16.
This study focused on evaluate the effect of homeopathic medicines on growth, survival, and modulation of the GIT microbiota in A. ventricosus.
MATERIALS AND METHODS
Organisms and maintenance. Juvenile A. ventricosus (1.98 ± 0.1 cm of average length) from Bahía de La Paz, Baja California Sur, México, were cleaned of epifauna with filtered and UV-sterilized seawater after arriving to laboratory and were acclimatised one week (22°C, 37 PSU salinity). During the bioassay, scallops were allocated in nursery upwellers with filtered and UV sterilised seawater and fed on microalgae Isochrysis galbana and Chaetoceros calcitrans (1:1) at 10.8% of dry weight of soft tissues/day (aproximatelly 2x108 cells org-1 d-1).
Homeopathic medicine formulation. Commercial medicines for human use or homeopathic products developed at Centro de Investigaciones Biológicas del Noroeste, S.C. (CIBNOR) from raw materials, were used in accordance with homeopathy procedures 15,16. Five treatments and three controls were applied in triplicate: T1 (ViP 1D + ViA 1D), T2 (ViP 7C + ViA 7C), T3 (AcF 1D + MsS 1D), T4 (PhA 7C + SiT 7C), T5 (ViT 31C), C1 (diluted ethanol 1:99), C2 (diluted/succussed ethanol 1C), and C3 (distilled water). Ethanol is the typical dilution-succussion vehicle 19, but it may trigger Phenoloxidase and Prophenoloxidase activity in shrimp 20 and increase hemocyte count in A. ventricosus21. To avoid potential side effects, ethanolic “stock” dynamisations (decimal or centesimal) were diluted and succussed in distilled water to prepare the next and final “experimental” homeopathic dynamisation.
Homeopathic components of T1 and T2 consisted in decimal or centesimal dynamisations (dilution/succussion), developed at CIBNOR from nosodic mother tinctures (MTs) of pathogenic and virulent strains of Vibrio parahaemolyticus (CAIM-170, ViP) and Vibrio alginolyticus (CAIM-57 ViA), related with high mortalities in bivalves and shrimp. Each MT was obtained from bacterial cultures at 1 x 108 CFU mL-1. Briefly, and in accordance to Mazón-Suástegui et al 16, the following procedures were applied: a) Bacterial cells were centrifuged (8000 g, 4°C, 20 min) and harvested from 15 mL of bacterial culture and washed twice; b) The corresponding pellets were diluted in 7.5 mL of MilliQ water and then inactivated through three freezing-thawing cycles (-80°C and 24°C, respectively) and sonicated eight times at 30 s, each time, to disrupt the cell wall and intracellular organelles; c) Unbroken cells were removed by centrifugation (3000 g, 4°C, 20 min) and the supernatant was diluted (1:1 v/v) in ethanol 87º GL (Similia® Mexico) and vortexed at 3200 rpm (BenchMixer®, Edison, NJ, U.S.A.) for two min to obtain 15 mL of MT from each bacterial strain.
The “stock” dynamisations (ViP 6C and ViPA 6C) were obtained by serial dilution and succussion in ethanol (1:99) of the respective TM. The “experimental” dynamisations (ViP 7C and ViA 7C) were obtained from respective 6C but substituting ethanol with distilled water.
The homeopathic treatment T3 (AcF 1D + MsS 1D) was the mixture of two decimal (1:9) dynamisations of 86% analytical grade phosphoric acid (Faga Lab®, Guamuchil, Sinaloa, Mexico) and a saturated solution of sodium metasilicate (Faga Lab®, Guamuchil, Sinaloa, Mexico). AcF´s TM was defined as the commercial product without dilution. MsS´s TM was prepared by diluting 9.4 g in 45 mL of distilled water, at 25°C. The first “experimental” decimal dynamisation (1D) of MsS was obtained by diluting 5 mL of MsS in 45 mL of distilled water (1:9) and vortexing two minutes at 3200 rpm (Benchmark mixer®, Benchmark Scientific Inc.). The “experimental” AcF 1D was obtained by the same procedure.
Treatments T4 (PhA 7C + SiT 7C) and T5 (ViT 31C) were prepared from commercial homeopathic medicines for human use (“stock” dynamisations). T4 was the next centesimal (1:99) dilution/succussion of Phosphoricum acid ® 6C and Silicea terra ® 6C (Similia®, Mexico) and T5 (ViT 31C), was the next 1:99 of VidatoX® 30C (Labiofam®, Habana, Cuba). The “experimental” dynamisations PhA 7C, SitT 7C and ViT 31C were prepared using distilled water.
Experimental design. We used 24 experimental units with 36 L of filtered and sterilized seawater and a continuous recirculating upflow pattern (1.68 mL s-1) during 21 h d-1. Each unit hatd 4 PVC cylinders with plastic mesh bottom, allocating 13 scallops cylinder-1 (52 scallops unit-1). Temperature and salinity were maintained at 23.5 ± 0.5°C and 37.5±0.5UPS, respectively. Organic waste was removed daily and siphoning up to 60% of the water to reduce environmental variations. The scallops were fed as previously described. The treatments were added directly to seawater for 21 days (100 μL L-1) stopping water and food flow for three hours to favour their absorption in epithelial and branchial tissues. At the end of the trial, growth and survival were recorded for each treatment and its three replicates, taking samples for GIT metagenomic studies.
Organic waste was removed daily by siphoning up to 60% of water volume to reduce environmental variations
Microbiota analysis. The bacterial DNA was extracted a two-time point: 24 samples at the beginning (T0) and 192 at the end (T21) of bioassay (8 scallops replicate-1; 24 scallops treatment-1). The GIT of each scallop was fixed individually (100 mg tissue in 500 µL RNA-Later®) and preserved at -20°C.
DNA Extraction and Illumina MiSeq sequencing. Tissue from the GIT of each scallop (216 samples) were homogenised using the homogeniser FastPrep, (MP Biomedicals®) at high speed (6 m s-1) for 30 s in 500 μL of buffer TE (50:50 Tris: EDTA, pH 8), followed by the addition of 20 μL of 10% SDS and incubation at 56°C for 30 min. Then, 250 μL of 7.5 M potassium acetate was added to lyse cells, and the mixture was incubated on ice for 15 min.
After centrifuging cell lysate at 8000 g at 4°C for 10 min, the water phase was extracted with phenol: chloroform: isoamyl alcohol (25:24:1) and centrifuged. The resultant water phase was extracted with chloroform: isoamyl alcohol (24:1) and centrifuged. Afterward, 350 μL of cold isopropanol was added to the supernatant, followed by overnight incubation at -20°C and DNA collection through centrifugation. The DNA pellet was washed twice with cold 70oGL ethanol, centrifuged, dried on air and resuspended in 20 μL of nuclease free water 22.
DNA pools of each replica were made after DNA extraction for a total of 27 samples. DNA concentration and quality were determined at A260 nm and A280 nm through NanoDrop spectrophotometer (NanoDrop 8000, Thermo Scientific®) and electrophoresis in 1.5% agarose gel using the Sybr Safe ® (Invitrogen) indicator. The total DNA of each individual sample, which was diluted in nuclease free water to a final concentration of 100 ng µL-1, was used to amplify ±700 bp of the hypervariable region V3-V5 of the 16S rRNA gen (positions 339 and 939 from Escherichia coli).
Amplification was performed with universal primers 357F (5′-CTCCTACGGGAGGCAGCAG-3′) 23 and CD[R] (5′-CTTGTGCGGGCCCCCGTCAATTC-3′) 24. The resultant PCR amplicons were sequenced through the 2x300 format of Illumina MiSeq sequencing platform of the National Laboratory for Genomic and Biodiversity (LANGEBIO-CINVESTAV), Guanajuato, Mexico.
Phylogenetic analysis. Sequences were processed using the classifier software RDP (Ribosomal Database Project) with an average sequencing quality (Phred quality score) > 30 and a similarity threshold (Confidence Cutoff) of 80%, able to classify sequences at genus level. The Greengenes 16S rRNA Database (http://greengenes.lbl.gov “Greengenes 16S”) was used for taxonomic classification at phylum, class, order, family, and genus, and only in some cases even at species level. Bioinformatics analysis was performed by LANGEBIO-CINVESTAV.
The software Estimates for Statistical analysis V.9.1.0 25 was used to calculate the Shannon-Weaver (H) and Simpson (J) indices of diversity as well as Chao richness estimates and to generate rarefaction curves. The software XLSTAT version 2016.05.33324 (Addinsoft 1995-2016) was used to compare microbial communities and treatments through the Principal Component Analysis (PCA) 26 and to detect differences on phylotype abundance and treatment using one-way analysis of variance (ANOVA) and Tukey´s multiple comparison test. Only were those principal components with high values (> 1.0) considered statistically significant.
Data accessibility. Sequences generated were uploaded to the NCBI Sequence Read Archive (SRA) with the key: Bioproject PRJNA341370, and the SRA access number: SRP089926.
RESULTS
Growth and Survival. No significant differences in weight growth rate (daily weight gain; g d-1) between HOM-treated and control groups were found, but significant differences in shell-length growth rate were detected (Figure 1A). The highest growth rate (p<0.05) corresponded to T1 (117 µm d-1) and T2 (108 µm d-1) against C1 (14 µm d-1), C2 (34 µm d-1) and C3 (20 µm d-1). Homeopathic treatments T3 and T5 exhibited the highest survival (p<0.05) (95% and 94%, respectively) when compared to control treatments C1 (88%) and C3 (85%), (Figure 1B).
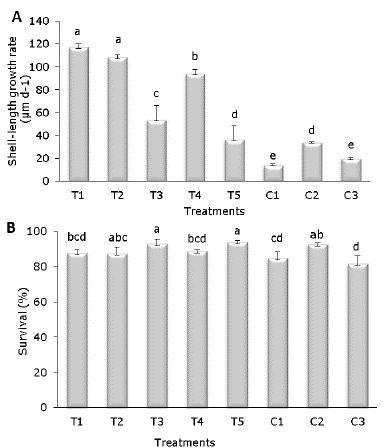
Figure 1 Growth rate (A) and survival (B) of A. ventricosus treated with homeopathic (HOM) medicines for 21 days. Values are mean ± confidence intervals at 95%. Different letter denotes significant differences (p<0.05).
Analysis of alfa diversity. PCR-amplified 16S-rRNA gene sequencing of microbiota associated to A. ventricosus GIT generated 21,717,584 reads with 80% classification from 27 amplicons. The number of OTUs was shown to be homogenous and had high richness with a number of OTUs/Chao-1 around 699/779 and 703/768 for treated and control groups, respectively, whereas the initial control group at the beginning of the experiment (T0), exhibited the highest value of 689/985 (Table 1). The Shannon-Weaver (H′) and Simpson (1-λ) indices also showed a high and homogeneous diversity for all experimental groups.
Table 1 Sequence analysis and alfa diversity estimation of the microbiota associated with A. ventricosus treated with homeopathic (HOM) medicines.
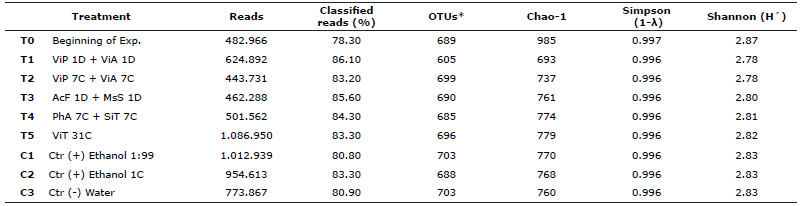
OTUs: Operational Taxonomic Units. *Database http://greengenes.lbl.gov “Greengenes 16S”
Although rarefaction curves did not reach the asymptotic phase, a clear separation and differentiation was detected between the rarefaction curves corresponding to HOM-treated groups and un-treated groups. As an exception, the rarefaction curve of the low (decimal) dynamisation HOM-treatment T1 (ViP 1D + ViA 1D) grouped together with T5 and control treatments (Figure 2). The groups C1, C2, C3, T1 and T5 reached the asymptotic phase whereas T0, T2, T3 and T4 did not clearly reach it; thus, a number of OTUs remained undetected for these groups.
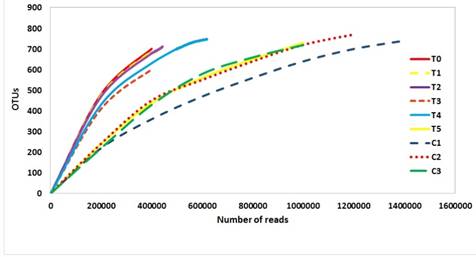
Figure 2 Rarefaction curves of the gastrointestinal tract (GIT) microbiota associated to homeopathic (HOM) treatments in juveniles of the marine scallop A. ventricosus.
Microbiota composition. The phylogenetic analysis showed the presence of 32 phyla, 68 classes, 134 orders, 295 families, 894 genera and 3241 species among all experimental groups. Microbiota composition associated to the GIT of A. ventricosus, based on a relative abundance > 1%, was dominated by the phyla Proteobacteria (≈29%), Actinobacteria (≈20%), Firmicutes (≈12%), Bacteroidetes (≈6.0%), Cyanobacteria (≈3.0%) and Chloroflexi (≈1.3%) (Figure 3). In general, all experimental groups had similar dominant phyla with significant differences (p≤0.05) for Proteobacteria, among T0 (34.9%) and T4 (26.1%), and for Actinobacteria, among T0 (22.9%) and T4 (18.7%) (Figure 3).
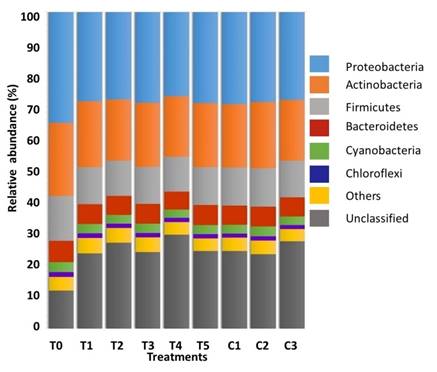
Figure 3. Relative abundance (%) of dominant Phyla (≥ 1%) in the GIT of A. ventricosus after treated with different homeopathic (HOM) medicines.
Proteobacteria was dominated by the classes α- (≈11%), β- (≈8%), γ- (≈6%, p≤0.001) and δ- (≈4%, p≤0.05). Significant differences between T0 and all other groups were recorded for the γ-Proteobacteria class. Significant differences were also detected between T0 and the treatments T2, T3 and T4 for the δ-Proteobacteria class (Figure 4).
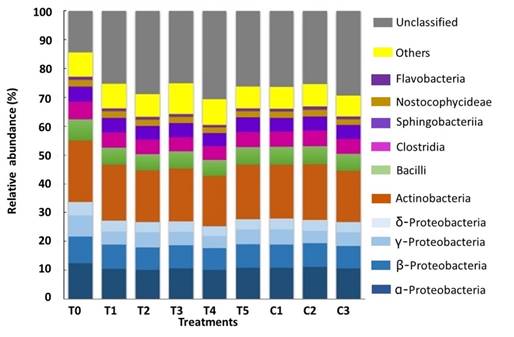
Figure 4 Relative abundance (> 1%) at class level, and Proteobacteria associated to the GIT of A. ventricosus treated with different homeopathic (HOM) medicines.
Symbiobacterium was the only genus among all dominant genera with significant differences (p ≤ 0.05) between HOM-treated scallops (T5, Highest) and Un-treated scallops at the initial of the bioassay (T0, Lowest). Other genera with dominant relative abundance of ≤ 1% included Microbacterium> Methylobacillus> Bacillus> Paenibacillus> Burkholderia> Nostoc> Methylobacterium> Leucobacter (Figure 5).
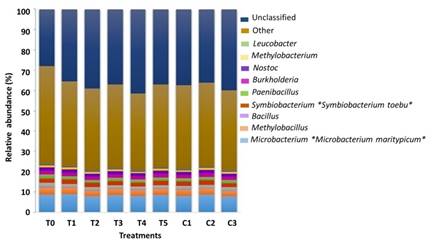
Figure 5 Bacterial relative abundance (> 1%) at genera and species level in the GIT microbiota associated to marine scallop A. ventricosus under HOM-treatments according to the analysis of variance (ANOVA) and Tukey´s multiple comparison test. (*) means significant difference p ≤ 0.05.
At species level, Microbacterium maritypicum (Actinobacteria) showed a greater relative abundance (p ≤ 0.05) in HOM-treated scallops receiving T1 and T3, against Un-treated T0. Symbiobacterium toebii (Firmicutes), was the other species whose abundance was significantly higher (p ≤ 0.05) in T5 and T2 with respect to T0 (Figure 5).
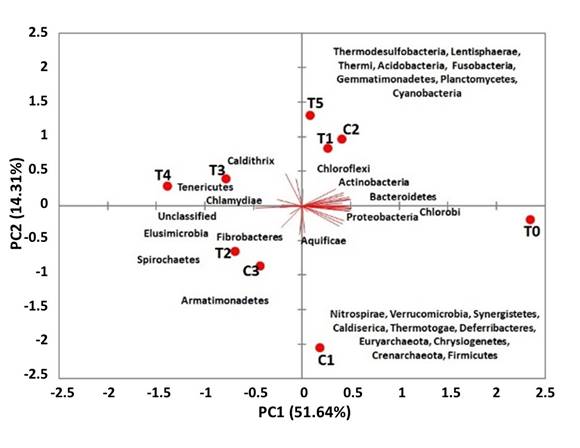
The variation on microbiota composition through PCA analysis of A. ventricosus treated with different homeopathy drugs may be explained by two principal components (PC1 = 51.64%; PC2 = 14.31%) with a cumulative variance of 65.95%. Treatments T2, T3, T4 and control C3 were found in the negative PC1 associated to the phyla Spirochaetes, Fibrobacteres, Tenericutes and Chlamydiae, and other less abundant phyla whereas T0, T1, T5 and the control C2 were found in the positive PC1 associated with Proteobacteria, Actinobacteria, Bacteroidetes, Firmicutes, Cyanobacteria and other less abundant phyla. These results agreed with the relative abundance and composition at Phyla level described above (Figure 6).
DISCUSSION
Diseases related to bacteria and viruses cause large mortalities and economic losses in global aquaculture industry and may even affect natural populations of molluscs, crustaceans, and fish. Bacteria are naturally present in the GIT microbiota of animals, including bivalve mollusc. Some species or strains are facultative pathogens that can attack susceptible animals. Pectinid molluscs, such as the Catarina scallop A. ventricosus feed by filtration and can become reservoir of potential pathogens transmissible to other organisms of the same or different species, including man 27.
In this study, the juvenile scallop treated with homeopathic nosodes T1 and T2 showed significant differences in shell-length-growth rate (μm d-1) with respect to control groups (p <0.05). The nosodes are widely used in homeopathy; they are broad-spectrum preparations prepared from biologic materials, such as cultures or clinical samples of microorganisms (e.g. bacteria, fungi and viruses), parasites, diseased tissues (cancerous tissues), or decomposition products from animals an human being 28.
Kiarazm et al 29 conducted a study in cows with subclinical mastitis with homeopathic medicines developed from nodules, resulting in a lower somatic cell count and a reduction in isolated bacteria compared with the control treatment. Camerlink et al 30 demonstrated that homeopathic agent Coli 30K is an attractive potential alternative in the prevention of E. coli diarrhoea in piglets. Mazón-Suástegui et al 16 demonstrated the effect of T2 on the immune and antioxidant response in Seriola rivoliana.
The greatest survival rate corresponded to the treatments T3 and T5 with respect to the control group C3. HOM-treatment T3 consisted in decimal dynamisations from sodium metasilicate and phosphoric acid TMs. In rats, sodium metasilicate has a beneficial action, because silica influences the complex network of cytokine interaction that regulates the immune response 31.
Nutrition is of most importance environmental factor which interact with other biological processes of life, and has a profound influence on several immune defense mechanisms. Deficient or excess levels of nutrients alter the immune system, and this reflects in measurable effects on several immune functions during in vivo or in vitro assays, but also it can modify infectious disease processes 32.
Homeopathic Phosphoric acid has the ability to treat gastrointestinal conditions related to malnutrition and poor assimilation of food; is used to promote the force of exhaustion and general vigor, and it has been related to the improvement of overall performance in A. ventricosus14. The sinergical interaction between sodium metasilicate and phosphoric acid in decimal (1D) homeopathic dilutions demonstrated to increase the immune response in juveniles of A. ventricosus; when this treatment was applied in a centesimal dynamisation (7C), it did not show a clear effect on immune response in Catarina scallop, but it increased the energetic reserves in mantle, muscle and digestive gland of the species, which are important energy storage tissues in mollusc bivalves 29.
T5 treatment is a commercial homeopathic medicine for human use (Vidatox®), whose active principle is the venom of the red scorpion Rhopalurus junceus, an endemic species of Cuba. Preclinical studies attribute antitumor properties 33) and according to Mazón-Suástegui et al 15, increased the survival and immunity of juvenile white shrimp Litopenaeus vannamei challenged with a pathogenic strain of Vibrio parahaemolyticus.
The microbiota associated with the GIT is a key factor to the development, immunity, and nutrition of the host 21. Most of those bacteria inhabits the surrounding environment 22 and are relevant to reduce host vulnerability against pathogens and their related infectious disease 22-24. In this study, the values of the Shannon index showed similar values in all the experimental groups from 2.75 to 2.83, which indicated that the microbiota associated with these organisms was diverse in all the groups.
A study by Trabal-Fernández et al 11 showed that microbiota associated to oyster spat (juvenile stage) was more diverse than those associated with adults of the species. In these sense, maturity of the GIT can influence the microbiota composition in marine organisms 34.
Proteobacteria was the dominant phylum in the GIT of A. ventricosus, both in the HOM-treated groups and in Un-treated controls, followed by Actinobacteria and Firmicutes. This Phylum was found predominant in early- and advanced juvenile and adult stages of the oysters C. corteziensis, C. gigas, and C. sikamea11; in larval stages of C. gigas35, C. virginica36, Haliotis discus37, Patella pellucida38 and the scallop Patinopecten yessoensisis39; as well as in fish and crustaceans such as Oncorhynchus mykiss and L. vannamei40.
Most classes within Proteobacteria plays important roles in bivalve molluscs, being able to degrade cellulose and agar which are major components of their food, and to fix nitrogen in bivalve GIT 41. Our results agree with Lasa et al 42 who reported Proteobacteria as the most abundant phylum in oyster. In this study, Actinobacteria was the second phylum in relative abundance. Some Actinobacteria produces secondary metabolites which can protect the host against infections 43 and play an important role in mineralization of organic matter, immobilization of mineral nutrients, nitrogen fixation and protection of the environment, in addition to their cellulolytic- 44 and chitinolytic activity 45.
Firmicutes was the third most abundant phylum (≈12%) in the GIT microbiota of A. ventricosus in all the experimental groups, with a lower relative abundance than the two previous phyla. Firmicutes is a highly relevant group in aquatic environments and found in the oyster microbiota 11, being involved in complex enzymatic processes, such as the degradation and fermentation of polysaccharides 46. It is dominant in the GIT of herbivorous organisms and some Lactobacillus species can stimulate the immune system, protecting the host from invasion and establishment of pathogens 47.
The dominant classes in this study were Alpha- and Betaproteobacteria; Alphaproteobacteria is dominant in marine environments 48 (Figure 4). Our results agreed with Trabal-Fernández et al 11, who reported Proteobacteria as the most abundant in oysters; however, Alpha- and Gammaproteobacteria were more abundant in juvenile oysters, while the most abundant class in adults was Gammaproteobacteria, followed by Beta- and Alphaproteobacteria.
The predominant genera in the GIT of juvenile A. ventricosus were Symbiobacterium and Microbacterium, followed by Methylobacillus, Bacillus, Paenibacillus, Burkholderia, Nostoc, Methylobacterium and Leucobacter, common in the marine environment 42. Symbiobacterium was dominant (p≤0.05) in the HOM T5 treatment (Vidatox®), which acts stimulating the immune system. This a very widespread genus in the natural environment, but its limited knowledge hinders its isolation 49. Oyster shells are common sources of Symbiobacterium sp. and other bacteria related to its growth 50. Ishii et al 51 inferred that this group of bacteria could play an important role in the nitrogen cycle in anaerobic environments, so they are beneficial because participates in nitrogen fixation, favoring the nutrition of marine organisms.
The species Microbacterium maritypicum was more abundant (p≤0.05) in T1 and T3 with respect to the controls. This bacterium produces siderophores and may inhibit the growth of opportunistic pathogens lacking the ability to produce iron 52. Iron is a limiting bioactive metal in seawater but essential for growth of marine bacteria 53, so these HOM-treatments may contribute to reduce pathogens.
HOM treatments T2 and T5 increased the abundance of Symbiobacterium toebii with respect to T0 (p≤0.05). Symbiobacterium species are symbiotic 54 but show a mono-marked growth if CO2 or bicarbonate is available 55. Joong-Jae et al 54 showed that Geobacillus kaustophilus, Escherichia coli and Bacillus subtilis had effects on the growth of S. toebii, indicating that there are growth promoting factors, widely present in various bacterial strains.
In conclusions the composition and diversity of the gastrointestinal microbiota of the marine mollusk A. ventricosus has been described for the first time, with dominance of the phylum Proteobacteria, Actinobacteria and Bacteroidetes. Homeopathic medication promoted growth and survival, but also the proliferation of Microbacterium maritypicum (T1, T3) and Symbiobacterium toebii. (T2, T5). This allows us to assume that aquaculture homeopathy has the potential to modulate that microbiota and improve seed production in the laboratory.
Acknowledgements
The study was funded by the Sectoral Fund for Research & Education of México: Project 258282 “Experimental evaluation of homeopathy and new probiotics in the cultivation of molluscs, crustaceans and fish of commercial interest” under the academic responsibility of JMMS. All the authors contributed to the manuscript, declare no conflict of interest and acknowledge CIBNOR technical staff: Delfino Barajas, Pablo Ormart, Julián Garzón, Guillermo García, Eulalia Meza. Diana Fischer edited the English manuscript.
REFERENCES
1. Ruíz-Verdugo CA, Koch V, Félix-Pico V, et al. Scallop Fisheries and Aquaculture in Mexico. In: Shumway S, Parsons GJ. Developments in Aquaculture and Fisheries Science, Vol. 40: Elseiver; 2016. p.1111-1125, 2016. DOI: https://doi.org/10.1016/b978-0-444-62710-0.00029-8. [ Links ]
2. Mazón Suástegui JM. Biología y cultivo de la almeja catarina Argopecten ventricosus (Sowerby II, 1842). [Tesis doctoral]. Barcelona (España): Universitat de Barcelona; 2005. [ Links ]
3. Mazón-Suástegui JM, Lodeiros Seijo C, Avilés-Quevedo A, Rodríguez-Jaramillo C, Ortíz-Cornejo N, Abasolo-Pacheco F. Cornstarch as a dietary supplement in conditioning broodstock and spat nursery of the Pacific calico scallop, Argopecten ventricosus. Lat Am J Aquat Res 2017; 45(5):915-921. DOI: http://dx.doi.org/10.3856/vol45-issue5-fulltext-6 [ Links ]
4. Oyinlola MA, Reygondeau G, Wabnitz CCC, Troell M, Cheung WWL. Global estimation of areas with suitable environmental conditions for mariculture species. PLoS ONE 2018; 13:e0191086. DOI: https://doi.org/10.1371/journal.pone.0191086 [ Links ]
5. Beaz-Hidalgo R, Balboa S, Romalde S, Figueras JL. Diversity and pathogenicity of Vibrio species in cultured bivalve mollusks. Environ Microbiol Rep 2010; 2:34-43. DOI: http://dx.doi.org/10.1111/j.1758-2229.2010.00135.x [ Links ]
6. Abasolo-Pacheco F, Campa-Córdova AI, Mazón-Suástegui JM, Tovar-Ramírez D, Araya R, Saucedo PE. Enhancing growth and resistance to Vibrio alginolyticus disease in catarina scallop (Argopecten ventricosus) with Bacillus and Lactobacillus probiotic strains during early development. Aquac Res. 2017; 48:4597-4607. DOI: https://doi.org/10.1111/are.13283 [ Links ]
7. Zheng D, Chang Q, Gao M, She Z, Jin C, Guo L, et al. Performance evaluation and microbial community of a sequencing batch biofilm reactor (SBBR) treating mariculture wastewater at different chlortetracycline concentrations. J Environ Manage. 2016; 182:496-504. DOI: http://dx.doi.org/10.1016/j.jenvman.2016.08.003 [ Links ]
8. Undabarrena A, Beltrametti F, Claverías FP, González M, Moore ERB, Seeger M and Cámara B. Exploring the Diversity and Antimicrobial Potential of Marine Actinobacteria from the Comau Fjord in Northern Patagonia, Chile. Front Microbiol. 2016; 19(7):1135. DOI: http://dx.doi.org/10.3389/fmicb.2016.01135 [ Links ]
9. Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest. 2014; 124(10):4212-4218. DOI: http://dx.doi.org/10.1172/JCI72333 [ Links ]
10. Rand-Weaver M, Margiotta-Casaluci L, Patel A, Panter GH, Owen SF, Sumpter JP. The Read-Across Hypothesis and Environmental Risk Assessment of Pharmaceuticals. Environ Sci Technol. 2013; 47:11384-11395 DOI: http://dx.doi.org/0.1021/es402065a [ Links ]
11. Trabal-Fernández N, Mazón-Suástegui JM, Vázquez-Juárez R, Ascencio-Valle F, Romero J. Changes in the composition and diversity of the bacterial microbiota associated with oysters (Crassostrea corteziensis, Crassostrea gigas and Crassostrea sikamea) during comercial production. FEMS Microbiol Ecol. 2014; 88(1):69-83. DOI: http://dx.doi.org/10.1111/1574-6941.12270 [ Links ]
12. Mente E, Gannonb AT, Nikoulia E, Hammerc H. Gut microbial communities associated with the molting stages of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture. 2016; 463(1):181-188. DOI: https://doi.org/10.1016/j.aquaculture.2016.05.045 [ Links ]
13. Mazón-Suástegui JM, Rosero-García A, Avilés-Quevedo A, Dumas S, Vega R, Rodríguez-Jaramillo C, Tovar-Ramírez D. Homeopathy for marine fish aquaculture: Increased growth and survival of juvenile spotted rose snapper Lutjanus guttatus. Homeopathy. 2016; 105:32-33. DOI: https://10.1016/j.homp.2015.12.055 [ Links ]
14. Mazón-Suástegui JM, García-Bernal M. Saucedo PE, Campa-Córdova AI, Abasolo-Pacheco F. Homeopathy outperforms antibiotics in juvenile scallop Argopecten ventricosus: Effects on growth, survival, and immune response. Homeopathy. 2017; 106: 18-26. DOI: https://doi.org/10.1016/j.homp.2016.12.002 [ Links ]
15. Mazón-Suástegui JM, García-Bernal M, Avilés-Quevedo A, Campa-Córdova A, Salas-Leiva J, Abasolo-Pacheco F. Assessment of homeopathic medicines on survival and antioxidant response in white shrimp Litopenaeus vannamei. Rev MVZ Córdoba. 2018; 23(3):6850-6859. DOI: https://doi.org/10.21897/rmvz.1373 [ Links ]
16. Mazón-Suástegui JM, Salas-Leiva J, Teles A, Tovar-Ramírez D. Immune and antioxidant enzyme response of Longfin yellowtail (Seriola rivoliana) juveniles to ultra-diluted substances derived from phosphorus, silica and pathogenic Vibrio. Homeopathy. 2019; 108(1):43-53. DOI: https://doi.org/10.1055/s-0038-1672197 [ Links ]
17. Ortiz-Cornejo NL, Tovar-Ramírez D, Abasolo-Pacheco F, Mazón-Suástegui JM. Homeopatía, una alternativa para la acuicultura. Revista Médica de Homeopatía. 2018; 10(1):18-24. DOI: https://doi.org/10.1016/j.homeo.2017.04.006 [ Links ]
18. Bellavite P, Conforti A, Ortolani R. Immunology and homeopathy. 3. Experimental studies on animal models. Evid Based Complement Alternat Med. 2006; 3(2):171-186. DOI: https://doi.org/10.1093/ecam/nel016 [ Links ]
19. Bellavite P, Signorini A. The emerging science of homeopathy: complexity, biodynamics, and nanopharmacology. 2nd ed Berkeley (CA): North Atlantic Books; 2002. [ Links ]
20. Hernández-López J, Gollás-Galván T, Vargas-Albores F. Activation of the prophenoloxidase system of the brown shrimp (Penaeus californiensis Holmes). Comp Biochem Physiol C. 1996; 113(1):61-66. DOI: https://doi.org/10.1016/0742-8413(95)02033-0 [ Links ]
21. Luna-González A, Maeda-Martínez AN, Vargas-Albores F, Ascencio-Valle F, Robles-Mungaray M. Phenoloxidase activity in larval and juvenile homogenates and adult plasma and haemocytes of bivalve molluscs. Fish Shellfish Immun. 2003; 15(4):275-282. DOI: https://doi.org/10.1016/S1050-4648(02)00165-1 [ Links ]
22. Hart ML, Meyer A, Johnson PJ, Ericsson AC. Comparative evaluation of DNA extraction methods from feces of multiple host species for downstream next-generation sequencing. PloS one. 2015; 10(11):e0143334. DOI: https://doi.org/ 10.7324/JABB.2019.70103. [ Links ]
23. Turner S, Pryerb KM, Mia VPM, Palmera JD. Investigating Deep Phylogenetic Relationships among Cyanobacteria and Plastids by Small Subunit rRNA Sequence Analysis. J Eukaryot Microbiol. 1999; 46(4):327-338. DOI: https://doi.org/10.1111/j.1550-7408.1999.tb04612.x [ Links ]
24. Rudi K, Skulberg OL, Larsen F, Jakobsen KS. Strain Characterization and Classification of Oxyphotobacteria in Clone Cultures on the Basis of 16S rRNA Sequences from the Variable Regions V6, V7, and V8. Appl Environ Microbiol. 1997; 63(7):2593-2599. https://aem.asm.org/content/63/7/2593 [ Links ]
25. Colwell RK, Elsensohn JE. EstimateS turns 20: statistical estimation of species richness and shared species from samples, with nonparametric extrapolation. Ecography 2014; 37:609 - 613. DOI: https://doi.org/10.1111/ecog.00814 [ Links ]
26. Krzanowski W. Principles of multivariate analysis: A User’s Perspective Vol. 23. 2nd ed. Oxford University Press; 2000. [ Links ]
27. Johnson C, Bowers J, Griffitt K, Molina V, Clostio R, Pei S, et al. Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington (United States). Applied and Environmental Microbiology. 2012; 78:7249-7257. DOI: https://doi.org/10.1128/AEM.01296-12 [ Links ]
28. Shah R. Scientific method of preparing homoeopathic nosodes. Indian J Res Homeopath. 2014; 8(3):166-173. DOI: https://doi.org/10.4103/0974-7168.141740 [ Links ]
29. Kiarazm M, Tajik P, Nava HG. Assessment of the effect of homoeopathic nosodes in subclinical bovine mastitis. Ann Biol Res. 2011; 2(5):552-562. https://www.semanticscholar.org/paper/Assessment-of-the-effect-of-homoeopathic-nosodes-in-Kiarazm-Tajik/7f86d1909a2ca8c21217c23da53ec8f65835b90b [ Links ]
30. Camerlink I, Ellinger L, Bakker EJ, Lantinga EA. Homeopathy as replacement to antibiotics in the case of Escherichia coli diarrhoea in neonatal piglets. Homeopathy. 2010; 99(1):57-62. DOI: https://doi.org/10.1016/j.homp.2009.10.003 [ Links ]
31. Nielsen FH. A novel silicon complex is as effective as sodium metasilicate in enhancing the collagen-induced inflammatory response of silicon-deprived rats. J Trace Elem Med.Bio. 2008; 22:39-49. DOI: https://doi.org/10.1016/j.jtemb.2007.11.004 [ Links ]
32. Costa V, Casamassimi A, Ciccodicola A. Nutritional genomics era: opportunities toward a genometailored nutritional regimen. J Nutr Biochem. 2010; 21(6):457-467. DOI: https://doi.org/10.1016/j.jnutbio.2009.10.012 [ Links ]
33. Díaz-García A, Morier-Díaz L, Frión-Herrera Y, Rodríguez-Sánchez H, Caballero-Lorenzo Y, Mendoza-Llanes D, et al. In vitro anticancer effect of venom from Cuban scorpion Rhopalurus junceus against a panel of human cancer cell lines. J Venom Res. 2013; 4:5-12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3717326/ [ Links ]
34. Kesarcodi WA, Philippe M, Jean-Louis N, Rene R. Protective effect of four potential probiotics against pathogen-challenge of the larvae of three bivalves: pacific oyster (Crassostrea gigas), flat oyster (Ostrea edulis) and scallop (Pecten maximus). Aquaculture. 2012; 344-349:29-34. DOI: https://doi.org/10.1016/j.aquaculture.2012.02.029 [ Links ]
35. Asmani K, Petton B, Le Grand J, Mounier J, Robert R, Nicolas JL. Establishment of microbiota in larval culture of Pacific oyster, Crassostrea gigas. Aquaculture. 2016; 464(1):434-444. DOI: https://doi.org/10.1016/j.aquaculture.2016.07.020 [ Links ]
36. King GM, Judd C, Kuske CR, Smith C. Analysis of Stomach and Gut Microbiomes of the Eastern Oyster (Crassostrea virginica) from Coastal Louisiana, USA. Plos One. 2012; 7(12):1-11. DOI: https://doi.org/10.1371/journal.pone.0051475 [ Links ]
37. Lee MJ, Lee JJ, Chung HY, Choi SH, Kima BS. Analysis of microbiota on abalone (Haliotis discus hannai) in South Korea for improved product management. Int J Food Microbiol. 2016; 234(3):45-52. DOI: https://doi.org/10.1016/j.ijfoodmicro.2016.06.032 [ Links ]
38. Dudek M, Adams J, Swain M, Hegarty M, Huws S, Gallagher J. Metaphylogenomic and Potential Functionality of the Limpet Patella pellucida’s Gastrointestinal Tract Microbiome. Int J Mol Sci 2014; 15(10):18819-18839. DOI: https://doi.org/10.3390/ijms151018819 [ Links ]
39. Sun X, Liu J, Li M, Zhao X, Liang J, Sun P, Ma, Y. Characterization of bacterial communities associating with larval development of Yesso Scallop (Patinopecten yessoensisis Jay, 1857) by high-throughput sequencing. J. Ocean Univ. China. 2016; 15(6):1067-1072. DOI: https://doi.org/10.1007/s11802-016-3092-8 [ Links ]
40. Zhang M, Suna Y, Liu Y, Qiaoa F, Chena L, Liub WT, Du Z, Li E. Response of gut microbiota to salinity change in two euryhaline aquatic animals with reverse salinity preference. Aquaculture . 2016; 454(1):72-80. DOI: https://doi.org/10.1016/j.aquaculture.2015.12.014 [ Links ]
41. Zeng S, Huang Z, Hou D, Liu J, Weng S, He J. Composition, diversity and function of intestinal microbiota in pacific white shrimp (Litopenaeus vannamei) at different culture stages. Peer J. 2017; 5:e3986. DOI: https://doi.org/10.7717/peerj.3986 [ Links ]
42. Lasa A, Mira A, Camelo-Castillo A, Belda-Ferre P, Romalde JL. Characterization of the microbiota associated to Pecten maximus gonads using 454-pyrosequencing. Int Microbiol. 2016; 19(2):93-99.DOI: https://doi.org/ 10.2436/20.1501.01.267 [ Links ]
43. Jensen PR, Moore BS, Fenical W. The marine actinomycete genus Salinispora: a model organism for secondary metabolite discovery. Nat Prod Rep. 2015; 32(5):738-751. DOI: https://doi.org/10.1039/c4np00167b [ Links ]
44. Subramani R, Aalbersberg W. Marine actinomycetes: an ongoing source of novel bioactive metabolites. Microbiol Res. 2012; 167(10):571-80. DOI: https://doi.org/10.1016/j.micres.2012.06.005 [ Links ]
45. Baier F, Copp JN, Tokuriki N. Evolution of enzyme superfamilies: comprehensive exploration of sequence−function relationships. Biochemistry. 2016; 55:6375-6388. DOI: https://doi.org/10.1021/acs.biochem.6b00723 [ Links ]
46. Dudek M, Adams J, Swain M, Hegarty M, Huws S, Gallagher J. Metaphylogenomic and Potential Functionality of the Limpet Patella pellucida’s Gastrointestinal Tract Microbiome. Int J Mol Sci . 2014; 15(10):18819-18839. DOI: https://doi.org/10.3390/ijms151018819 [ Links ]
47. Nayak SK. Role of gastrointestinal microbiota in fish. Aquac Res 2010; 41(11):1553-1573. DOI: https://doi.org/10.1111/j.1365-2109.2010.02546.x [ Links ]
48. Ashraf R, Shah NP. Immune system stimulation by probiotic microorganisms. Critical reviews in food science and nutrition. 2014; 54(7):938-956. DOI: https://doi.org/10.1080/10408398.2011.619671 [ Links ]
49. Yilmaz P, Yarza P, Rapp JZ, Glöckner FO. Expanding the world of marine bacterial and archaeal clades. Front. Microbiol. 2016; 6:1524. DOI: https://doi.org/10.3389/fmicb.2015.01524 [ Links ]
50. Sugihara T, Watsuji TO, Kubota S, Yamada K, Oka K, Watanabe K, Meguro M, Sawada E, Yoshihara K, Ueda K, Beppu T. Distribution of Symbiobacterium thermophilum and related bacteria in the marine environment. Biosci Biotechnol Biochem. 2008; 72(1):204-211. DOI: https://doi.org/10.1271/bbb.70619 [ Links ]
51. Ishii S, Yamamoto M, Kikuchi M, Oshima K, Hattori M, Otsuka S, Senoo K. Microbial populations responsive to denitrification-inducing conditions in rice paddy soil, as revealed by comparative 16S rRNA gene analysis. Appl Environ Microbiol . 2009; 75(2):7070-7078. DOI: https://doi.org/10.1128/AEM.01481-09 [ Links ]
52. Sugita H, Mizuki H, Itoi S. Diversity of siderophore-producing bacteria isolated from the intestinal tracts of fish along the Japanese coast. Aquacult Res. 2012; 43(2): 481-488. DOI: https://doi.org/10.1111/j.1365-2109.2011.02851.x [ Links ]
53. Boyd PW, Ellwood MJ. The biogeochemical cycle of iron in the ocean. Nat Geosci 2010; 3(10):675. DOI: https://doi.org/10.1038/ngeo964 [ Links ]
54. Joong-Jae K, Ryoji Masui, Seiki Kuramitsu, Jin-Ho Seo, Kwang Kim, Moon-Hee S. Characterization of Growth-supporting Factors Produced by Geobacillus toebii for the Commensal Thermophile Symbiobacterium toebii. J Microbiol Biotechnol 2008; 18(3):490-496. https://www.ncbi.nlm.nih.gov/pubmed/18388467 [ Links ]
55. Watsuji TO, Kato T, Ueda K, Beppu T. CO2 supply induces the growth of Symbiobacterium thermophilum, a syntrophic bacterium. Biosci Biotechnol Biochem . 2006; 70(3):753-756. DOI: https://doi.org/10.1271/bbb.70.753 [ Links ]
Received: March 2019; Accepted: June 2019; Published: September 2019











 text in
text in