Introduction
In 2017, the World Health Organization (WHO) produced a global priority pathogens list (global PPL) of antibiotic-resistant bacteria, in order to prioritize the development of an alternative antibiotic therapy for all pathogens.
In total, there are 12 pathogens on this list. They are divided into three categories: priority 1(critical), priority 2(high) and priority 3(medium) 1.
The problematic of drug-resistant bacteria is serious. It causes 700,000 deaths per year and would become an extremely disturbing 10 million per year in 2050 if there are no policies put in place to stop the worrying spread of Antimicrobial Resistance (AMR) 2.
In the United States, methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum β-lactamase (ESBL) infections are common. The national estimated number of cases for MRSA in 2017 was 323,718 (95% confidence interval [CI], 287,967-359,469). The number of incident case for ESBL in 2017 was 197,378 (95%CI, 173,913-220,842) 3
In Europe, a study from the European Antimicrobial Resistance Surveillance Network (EARS-Net data) estimated that 148727 cases (95% uncertainly interval [UI] 131757-166361) of infections and 7049 cases (95%UI6308-7863) of deaths attributable to MRSA occurred in 2015 in the EU and in the European Economic Area (EEA) 4.
In Japan, the rate of resistance to carbapenems in Escherichia coli and Klebsiella pneumoniae has remained low at less than 1%, and the proportion of MRSA in Staphylococcus aureus is around 50% 5.
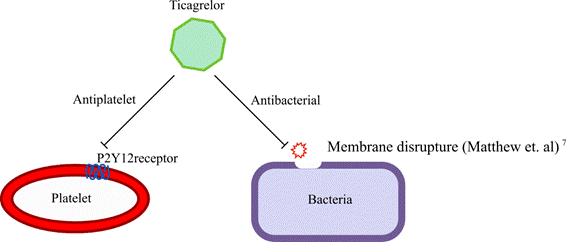
Despite the lack of alternatives to fight against these resistant bacterias, recently there have been reports that ticagrelor, an antiplatelet drug, also displays antibacterial activities 6)(7)(8)(9. Figure 1 describes the mechanism of action of Ticagrelor:
The purpose of this review is to analyze articles and case reports that describe the antimicrobial action of ticagrelor.
Method
We searched all the articles from PubMed, using the following keywords: “Ticagrelor + Antibacterial”, “Ticagrelor + Antimicrobial”, and “Ticagrelor + Bactericide”. The search was restricted to articles with results of laboratory experiments such as blood cultures, pharmacological chemistry reports and retrospective results analyses. The articles were searched by selecting all articles in English. All the articles were first checked as to whether they are related to the antimicrobial activity, and not to the original antiplatelet function.
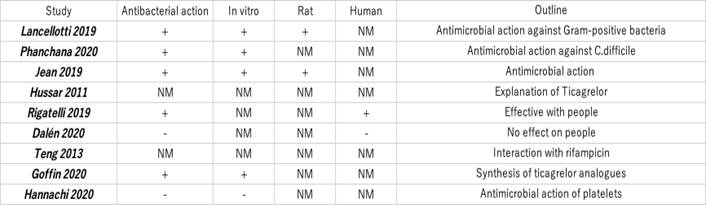
Of the 9 papers selected, one describes the properties of ticagrelor 13, one describes the interaction between ticagrelor and rifampicin 14, five of the remaining papers mention antibacterial activity 6)(7)(8)(9)(11 and two report no antibacterial activity 10)(12.
Results
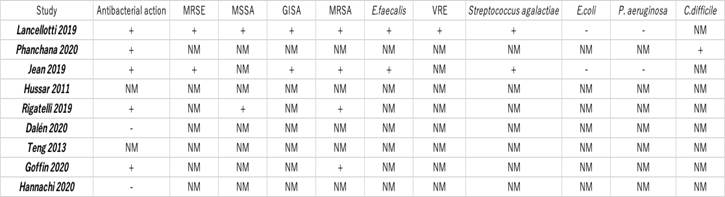
In vitro, ticagrelor and AR-C124910 (ticagrelor’s major metabolites) had bactericidal activity against Gram-positive strains tested, including drug-resistant strains glycopeptide-intermediate Staphylococcus aureus (GISA), methicillin-resistant Staphylococcus epidermidis (MRSE), MRSA, and vancomycin-resistant Enterococcus faecalis (VRE). The minimal bactericidal concentration (MBC) was of 20μg/mL against methicillin-sensitive Staphylococcus aureus (MSSA), GISA, MRSA, and VRE; 30μg/mL against MRSE; and 40μg/mL against E. faecalis and Streptococcus agalactiae. Ticagrelor inhibited MRSA, MRSE, and VRE biofilm formation in a dose-dependent manner 6.
In mice, conventional oral antiplatelet dosages of ticagrelor (3 mg/kg loading dose, then 1.5 mg/kg twice daily) inhibited the biofilm growth on S. aureus-infected implants and the dissemination of bacteria to surrounding tissues 6.
In addition, ticagrelor exhibited bactericidal activity against C. difficile, a Gram-positive, spore-forming bacterium. Ticagrelor exhibited a minimum inhibitory concentration (MIC) below 40μg/mL for all ribotypes used in the experiment 7.
In experiments that administered ticagrelor to humans, a significant difference in all-cause mortality was observed among ST-segment elevation acute myocardial infarction (STEMI) patients treated with ticagrelor and clopidogrel in both MSSA (2.8% vs 14.2%, p = 0.004) and MRSA (3.8% vs 15.9%, p = 0.04) groups 8.
Dalén et al. researched 2311 patients treated with ticagrelor or clopidogrel within 14 days prior to isolated primary CABG, of which 1293 patients (55.9%) received clopidogrel and 1018 patients (44.1%) received ticagrelor. The propensity score matched analyses, showing that ticagrelor had a similar incidence of infectious complications as clopidogrel 10.
Tables 1 and 2 present the total number of articles reviewed:
Discussion
There are in total 12 pathogens on the global PPL, which are divided into three categories: priority 1(critical), priority 2(high) and priority 3(medium). Priority 1 (critical) includes the carbapenem-resistant Acinetobacter baumannii, the carbapenem-resistant Pseudomonas aeruginosa, and the carbapenem-resistant 3rd generation cephalosporin-resistant Enterobacteriaceae.
Priority 2 (high) includes the vancomycin-resistant Enterococcus faecium, the methicillin-resistant, vancomycin intermediate and resistant Staphylococcus aureus, the clarithromycin-resistant Helicobacter pylori, the fluoroquinolone-resistant Campylobacter, the fluoroquinolone-resistant Salmonella spp., and the 3rd generation cephalosporin and fluoroquinolone-resistant Neisseria gonorrhoeae. Priority 3 (medium) includes penicillin-non-susceptible Streptococcus pneumoniae, ampicillin-resistant Haemophilus influenzae, and fluoroquinolone-resistant Shigella spp1.
In the United States, the national estimation of incident cases for MRSA, VRE and Extended-spectrum cephalosporin resistance in Enterobacteriaceae is suggestive of extended-spectrum beta-lactamase (ESBL) production. Carbapenem-resistant Enterobacteriaceae, carbapenem-resistant Acinetobacter species, and Multidrug-resistant (MDR) Pseudomonas aeruginosa give a total of 622,390 cases (95%CI, 579,125-665,655). A total of 517,818 infections (83%) had the infection origin in the community, and 104,572 infections (17%) had originated intra hospital. MRSA and ESBL infections were common and together accounted for 84% of all the cases in 2017 (52% for MRSA and 32% for ESBL infection) 3.
In Europe, from EARS-Net data collected between Jan 1, 2015, and Dec 31, 2015, a study estimated that there were 148727 (95% UI 131757-166361) cases of infections and 7049 (95%UI6308-7863) cases of deaths were attributable to MRSA 4.
For Gram-negative bacteria like E.coli and Klebsiella pneumoniae, the rising resistance to carbapenem has become a problem in many countries in the past decade.
In Japan, the rate of resistance to carbapenems of E. coli and Klebsiella pneumoniae is low. However, for Gram-positive bacteria, the proportion of MRSA in Staphylococcus aureus is around 50%, which is high, although declining in recent years. For Enterococcus spps., vancomycin-resistant E. faecalis occurs in less than 0.05% of cases, and vancomycin-resistant E. faecium occurrences are relatively low at 1.5% of reported cases 5.
Different to thienopyridines, which is irreversible when it binds to the P2Y12 receptor, ticagrelor (AZD6140) is the first drug that binds to P2Y12 receptor reversibly antagonizing, blocking the platelet aggregation through ADP-inducing. This binding exhibits rapid onset and offset of effect, which closely follow drug exposure levels. Furthermore, ticagrelor does not require metabolic activation 15.
Ticagrelor is indicated to reduce the rate of thrombotic events in cardiovascular patients with acute coronary syndrome (ACS) even after procedures such as the percutaneous coronary intervention (PCI) 13.
The PLATO trial compared ticagrelor versus clopidogrel in patients with Acute Coronary Syndrome. It showed that ticagrelor had significantly reduced in the rate of deaths caused by myocardial infarction or stroke 16.
The post hoc analysis of the PLATO study showed that the mortality risk following a pulmonary adverse event (PAE) and sepsis in Acute Coronary Syndrome patients appears to be lower with ticagrelor administration compared to patients treated with clopidogrel 17.
Travis et al. demonstrate that targeting the platelet P2Y12 receptor reduces platelet-leukocyte interactions, modifies the inflammatory biomarkers, and improves the lung function in the context of pneumonia in experimental sepsis models 18.
Lancellotti et al. researched whether ticagrelor or its metabolites could possess antimicrobial properties. The experiment in vitro found that ticagrelor and AR-C124910(ticagrelor’s major metabolites) had bactericidal activity against Gram-positive bacteria, including drug-resistant strains such as GISA, MRSE, MRSA, and VRE. The minimal bactericidal concentration was 20μg/mL for MSSA, GISA, MRSA, and VRE; 30μg/mL for MRSE; and 40μg/mL for E. faecalis and S. agalactiae6.
In contrast to the paper mentioned above, Nadji et al. stated that ticagrelor has no antimicrobial effect in vivo. The experiment results describe platelets and antimicrobial action and observed the antimicrobial effect of ticagrelor as a control. The concentration of ticagrelor used in this study was 10 µM(mol/L) 12 but the minimum effective concentration of ticagrelor had not been reached 6, explaining the discrepancy in results between this study and others.
A subminimal bactericidal concentration of ticagrelor (10μg/mL) combined with vancomycin (4μg/mL) reduced approximately 50% of the initial MRSA inoculum, depicting synergistic activity (6). Ticagrelor also increased the bactericidal activity of rifampicin, ciprofloxacin, and vancomycin in a disk diffusion assay 6.
Furthermore, the studies suggested that ticagrelor is volume dependent 6. Ticagrelor inhibited MRSA, MRSE, and VRE biofilm formation in a dose-dependent manner: the biofilm mass was reduced by more than 85% after exposure to 20μg/mL of ticagrelor. In mice, conventional oral antiplatelet dosages (3 mg/kg initial dose, then 1.5 mg/kg twice daily) inhibited biofilm growth on S. aureus preinfected implants and dissemination of bacteria to the surrounding tissues 6.
Lancellotti et al. did not isolate bacteria resistant to ticagrelor, and serial passaging of MSSA or MRSA in the presence of subinhibitory concentrations of ticagrelor did not create for resistant mutants as opposed to ofloxacin or rifampicin, which is reassuring for long-term antiplatelet usage 6.
Although bactericidal concentrations are not reached systemically in patients receiving typical dosages for treating cardiovascular diseases (ticagrelor Cmax = 1.2μg/mL after one 180mg loading dose and 0.75μg/mL at 90 mg twice daily steady state), antibacterial activity at infection sites may still be achieved through local, possibly platelet-driven, drug accumulation 6.
Furthermore, ticagrelor exhibited bactericidal activity against C. difficile, which is a spore-forming bacterium. Ticagrelor exhibited a MIC of 40μg/mL for ribotype 012(630), 017, 020, 023, 046, 056. 095, 126, excepting ribotypes 027 (strain R20291), 106, and 117, which have had a MIC of 20-40μg/mL 6.
Ticagrelor reduced spore germination of C. difficile. Increasing the concentration of ticagrelor to 40μg/mL substantially hindered the germination rate, suggesting a dose dependent action of ticagrelor on spore inactivation 6.
Although the MICs of ticagrelor were markedly higher than metronidazole and vancomycin (ticagrelor:20-40μg/ml, metronidazole:0.25-1μg/ml, vancomycin:0.0625-1μg/ml), the same range of MIC of ticagrelor was observed in all strains of C. difficile tested regardless of their sensitivity background to metronidazole and vancomycin 7.
For gram-negative bacteria, ticagrelor was ineffective against E. coli and Pseudomonas aeruginosa in concentrations up to 80μg/mL 6.
In human administration, a significant difference for all mortality cause was observed among patients treated with ticagrelor and clopidogrel in both MSSA (2.8% vs 14.2%, p = 0.004). and MRSA groups (3.8% vs 15.9%, p = 0.04), respectively. This is why the potential antibacterial benefit is suggested in patients treated with ticagrelor who had a MSSA or MRSA infection after a primary PCI in ST-segment elevation acute myocardial infarction (STEMI) 8.
In Magnus et al.’s study, out of 2311 patients, 1293 (55.9%) received clopidogrel and 1018 (44.1%) received ticagrelor preoperatively. In all analyses, ticagrelor was associated with a similar incidence of infectious complications compared to clopidogrel. Patients of this study were all adult patients who were preoperatively treated with ticagrelor or clopidogrel within 14 days prior to isolated primary coronary artery bypass grafting (CABG) from January 2015 to May 2017 10.
This discrepancy of results between Rigatelli 8 et al.’s and Dalén 10 et al.’s research may be due to the difference of the types of involved bacteria. The study of Rigatelli et al. was limited to MRSA and MSSA (8), and Dalén et al.’s study was limited to interventional procedure without specifying the type of bacteria 10. In post-CABG infections, Pseudomonas aeruginosa and other Gram-negative organisms have been shown to be related 19.
The Lancellotti et al. study has shown that ticagrelor was ineffective against Gram-negative strains in concentrations up to 80 μg/mL in vivo 6.
This suggests that the use of ticagrelor for MRSA and MSSA is likely to be effective in humans.
The antimicrobial action of ticagrelor is not completely understood. Eric et al. conducted a study to identify the structural elements responsible for the antiplatelet and antimicrobial activities of ticagrelor metabolites and simplified structures and found that only ticagrelor and its main metabolite AR-C124910 showed antibacterial activity against MRSA. Slight modifications of the structure of ticagrelor led to a dramatical loss of the antibacterial activity against MRSA, while the antiplatelet activity was maintained with some simplified ticagrelor analogues. These results indicate that the antiplatelet and antibacterial activity of ticagrelor were not necessarily linked and may be due to distinct mechanisms 11.
Conclusion
Ticagrelor showed antibacterial activity against Gram-positive bacteria, including C. difficile and other drug-resistant bacteria. In contrast, ticagrelor had no antimicrobial activity against Gram-negative bacteria. There is currently no clear explanation for the antibacterial effect of ticagrelor. The elucidation of the mechanism of antibacterial activity is of great importance for its use as an antibacterial agent. Further studies are needed to evaluate the effect of ticagrelor.