Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería y Desarrollo
Print version ISSN 0122-3461On-line version ISSN 2145-9371
Ing. Desarro. no.26 Barranquilla July/Dec. 2009
ARTÍCULO CIENTÍFICO / RESEARCH ARTICLE
Evaluation of the titanium dioxide photocatalysis for the degradation of a commercial pesticides mixture
Evaluación de la fotocatálisis con dióxido de titanio en la degradación de una mezcla de pesticidas comerciales
José Colina-Márquez* Luis Zuluaga** Fiderman Machuca Martínez***
* Ingeniero Químico, M.Sc., candidato a Doctor en Ingeniería. Grupo de Investigación en Tecnologías Avanzadas de Oxidación GAOX, Escuela de Ingeniería Química, Universidad del Valle, Cali (Colombia). jocolina@univalle.edu.co
** Ingeniero Químico. Grupo de Investigación en Tecnologías Avanzadas de Oxidación GAOX, Escuela de Ingeniería Química, Universidad del Valle, Cali (Colombia). lzuluaga@univalle.edu.co
*** Ingeniero Químico, M.Sc. Ph.D., Profesor Escuela de Ingeniería Química, Grupo de Investigación en Tecnologías Avanzadas de Oxidación GAOX, Universidad del Valle, Cali (Colombia). fiderman@univalle.edu.co
Correspondencia: Calle 13 No 100 - 00, Universidad del Valle, Ciudad Universitaria de Meléndez, Edificio 336, piso 2, Of. 2027, A. A. 25360, Cali (Colombia).
Grants and support: Authors thank Colciencias and Univalle for the financial support (Grant 110647922029 and 2565) for the development of this research, Colina-Marquez and Machuca-Martínez thank to Colciencias by the scholarships of their Ph.D. studies.
Fecha de recepción: 4 de abril de 2009
Fecha de aceptación: 21 de septiembre de 2009
Abstract
In this work, the adsorption and reuse capability of TiO2 were studied. The photocatalytic degradation of a commercial pesticide mixture consisting of Karmex®, Profiamina®, Igram®, and the coadjuvants Cosmoaguas® and Inex-A®, was evaluated. As experimental setup, a set of batch reactors and UV artificial light were used. The reduction of total organic carbon (TOC) was measured to evaluate the mineralization capability of the photocatalytic process. The optimal conditions were found by using a response surface experimental design. Three consecutive catalyst reuses were made with no special rinse treatment. The catalyst lost its capability to adsorb and degrade the pollutant mixture after three reuses because of the desorption of the intermediate species produced during the photocatalytic process.
Keywords: Heterogeneous photocatalysis, titanium dioxide, adsorption equilibrium, atrazine, ametryne, 2,4-D.
Resumen
En este trabajo se estudió la capacidad de adsorción y reutilización del TiO2. Se evaluó la degradación fotocatalítica de pesticidas comerciales, consistente en Karmex®, Profiamina®, Igram®, y los coadyuvantes Cosmoaguas® y Inex-A®. Como arreglo experimental se usó un conjunto de reactores por lotes y luz artificial UV. Se midió la reducción de carbón orgánico total (COT) para evaluar la capacidad de mineralización del proceso fotocatalítico. Las condiciones óptimas se encontraron usando un diseño experimental de superficie de respuesta. Se llevaron a cabo tres reutilizaciones consecutivas del catalizador sin hacer lavados o algún tratamiento adicional. El catalizador perdió su capacidad de adsorber y degradar la mezcla contaminante después de las tres reutilizaciones debido a la desorción de las especies intermedias producidas durante el proceso fotocatalítico.
Palabras clave: Fotocatálisis heterogénea, dióxido de titanio, equilibrio de adsorción, atrazina, ametrina, 2,4-D.
1. INTRODUCTION
Heterogeneous photocatalysis has been used for treatment of several pollutants such as pesticides [1]. These substances have low or no biodegradability; therefore, an advanced oxidation process is suitable for their treatment. Previous works have studied heterogeneous and homogeneous solar photocatalysis with pilot scale photoreactors [2], [3].
Pesticides are used in diverse crops and agricultural activities worldwide. There are several kinds of pesticides depending on the chemical structures of their functional groups. Many studies about TiO2-based heterogeneous photocatalysis have been carried out with carbamates, organochlorinated, organophosphates, triazines, and phenylureas [4] - [6].
To evaluate the TiO2-based photocatalysis as an alternative treatment for pesticide residues, it is necessary to know about the adsorption and reuse capabilities of the selected catalyst. The time that is required to achieve adsorption equilibrium is very important in photocatalytic research, since the actual photocatalytic work can be assessed for the selection of design and process variables. This information is quite relevant for larger scale applications of this kind of processes, due to the high incidence of reuse in the operating costs of the photocatalytic plant. The TiO2 reuse has been studied at lab scale by the evaluation of the photocatalytic degradation of chlorophenol and 1,2 dichlorophenol [7], and the pH and catalyst load effects were also studied. The reuse was also studied on photodegradation of dyes [8]. The adsorption equilibrium on the catalyst surface, and the catalyst capability for consecutive reuses, depend on the substrate nature and the reaction conditions. Although there is a large quantity of papers related to photocatalytic degradation of pesticides, a lack of studies about catalyst reuse on a specific mixture of these substances is evident, because of the large variety of commercial mixtures used in diverse agricultural applications and world regions.
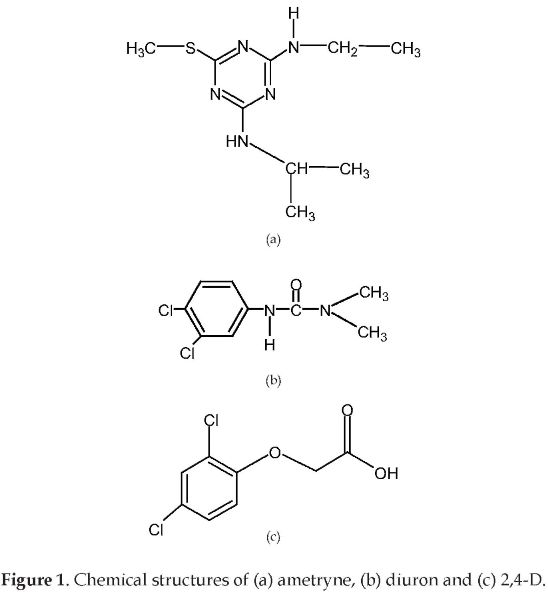
In this work, the adsorption and reuse capabilities of the Aeroxide P-25® (titanium dioxide manufactured by Degussa) were evaluated for the photocatalytic degradation of a commercial pesticide mixture used in sugar cane crops in Colombia. The selected pesticides were: Profiamina®, Igram®, and Karmex®, whose main active compounds are: 2,4 dichlorophenoxiacetic acid (2,4-D), ametryne, and diuron, respectively. Figure 1 shows the chemical structures of these substances. 2,4-D belongs to the organochlorinated group, whereas the ametryne is an atrazine, and the diuron, a phenylurea. Furthermore, Inex-A® and Cosmoaguas® were added to the mixture. These substances are tensoactive agents and surfactant-based compounds (long aliphatic chains with hydrophilic groups at the ends). An experimental set of tests was aimed at obtaining the necessary time to achieve the adsorption equilibrium. Lastly, the photocatalytic degradation was evaluated by using batch reactors and an artificial UV light source. The optimal operating conditions for the photocatalytic degradation were found by a response surface methodology. The catalyst reuse tests were carried out at the found conditions, with no special rinse treatment for the catalyst.
2. METHODOLOGY Materials
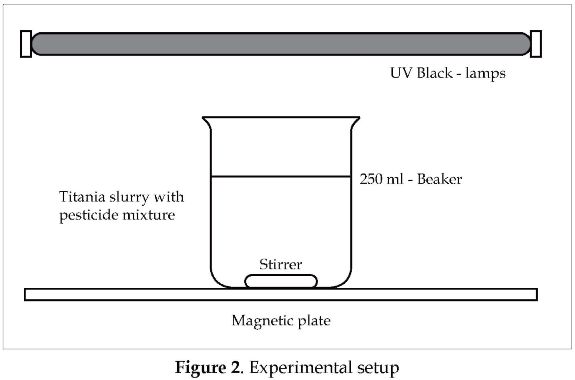
The pesticides selected for this study were: Profiamina®, Ingram®, and Karmex ®; and the aditives: Inex-A®, and Cosmoaguas®. The catalyst was Aeroxide P-25® (manufactured by Degussa), and it was used as received. For the photocatalytic degradation, 150-ml beakers were used as batch reactors, and five black-light Opalux® lamps (with 25 W of nominal power) supplied the UV radiation for all the tests. The radiation intensity was measured with an Acadus S85 radiometer, and its value was 25 W/m2. The temperature during all the tests was 30.5 °C. A Variomag® magnetic plate was used to stir the TiO2-slurries. All the components of the experimental setup were located inside a box of 90 cm x 40 cm x 30 cm, as shown in Figure 2.
Procedure
A 1-L slurry was prepared with 16.7 g of Karmex®, 10 ml of Profiamina®, 10 ml of Igram®, 1 ml of Inex-A®, and 0.7 g of Cosmoaguas®. A 35 ml-aliquot of this solution was diluted with 5 L of distilled water.
Adsorption tests
A Shimadzu 5050 TOC analyzer was used to measure the TOC for each run sample. These concentrations were useful to determine the adsorption equilibrium time, and the catalyst capabilities for degradation and reuse. The run time for adsorption tests was 60 minutes under darkness, at the natural pH of the slurry. The catalyst concentrations used for these experimental runs were 0.3 and 0.8 g/L.
Photodegradation tests
The optimal operating conditions were found by statistically analyzing the results obtained with a full-composite experimental design. The variables studied were: initial pH, catalyst load, and mineralization. The initial pH was adjusted with HCl 0.2N or NaOH 1N. The time for each run was 90 minutes. The photodegradation of pesticide mixture was calculated from TOC reduction. The samples obtained underwent a 24-hours sedimentation for later catalyst separation [9].
Reuse tests
The loss of activity of TiO2 catalyst was determined from the reduction of mineralization capability after four consecutive reuses. The TOC concentration was measured at the beginning and the end of each reuse test. The conditions used for these experimental runs were the optimal ones found in the full-composite experimental design mentioned above. Several reactors were used for each run to avoid larger volume changes. The time for each run was 120 minutes. Once the catalyst had been separated by decantation, it was recovered and dried at 130°C, with no further treatment or rinsing process. IR analysis was used to determine the representative families of chemical intermediates adsorbed on the catalyst surface after the reuses [7].
3. RESULTS AND DISCUSSION
TOC adsorption on the catalyst surface
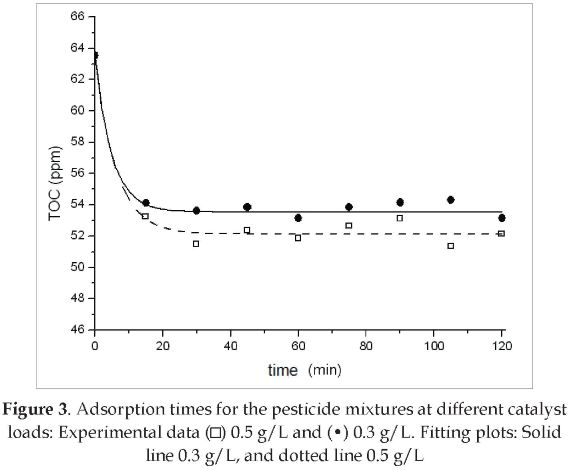
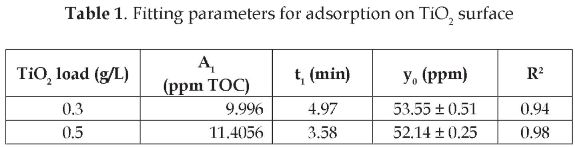
The plots obtained from fitting experimental data to the parameter model given in Eq. (1), resulted in decay type curves, as shown in Figure 3.
The values of the parameters obtained from the two plots are listed in Table 1. From these plots, it follows that the TOC achieves the adsorption equilibrium after 20 minutes of continuous stirring under darkness conditions. Previous works have reported adsorption equilibrium times from 15 minutes [10] to 60 minutes [4]. The difference between the results of these two studies is due to the fact that they were carried out with different pesticides. This indicates that the pesticide nature is relevant in the adsorption phenomenon. Similarly, it was observed in this study that the catalyst load had a positive effect on the adsorption. This was because of the larger catalyst surface available for adsorption.
Photocatalytic degradation
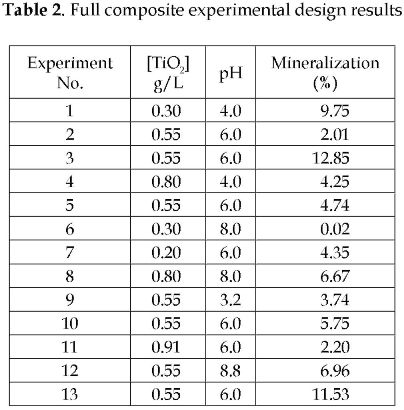
The photocatalytic degradation results were from 0.02 to 12.85%, as shown in Table 2. With the help of the Minitab 14® software, the optimal operating conditions of the photocatalytic degradation were found. For this study, the optimal catalyst load was 0.25 g/L, and optimal initial pH was 3.17.
The optimal catalyst concentration was under the considered interval (0.3 - 0.8 g/L). Higher TiO2 load had a negative effect on the photocatalytic degradation due to the higher slurry turbidity, which did not allow the penetration photons of the reactor inner zones.
The photocatalytic degradation was favored by the acidic initial pH. This positive effect of the acidic medium is related to the ionic nature of the substrate and the intermediate produced during the reaction. These substances are adsorbed better or worse on the catalyst surface depending on the electric affinity to the surface charges. For a pH lower than the zero-charge pH of TiO2 (pHzc = 6.8), [11] the catalyst surface is positively charged; therefore, it means that the intermediates produced are anionic species.
Catalyst reuse
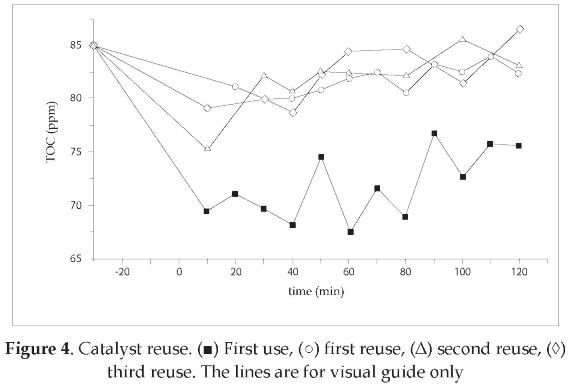
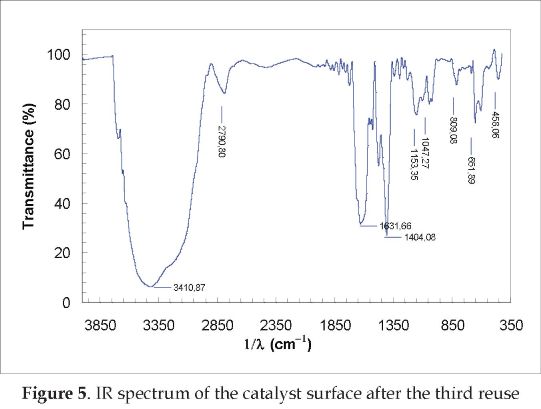
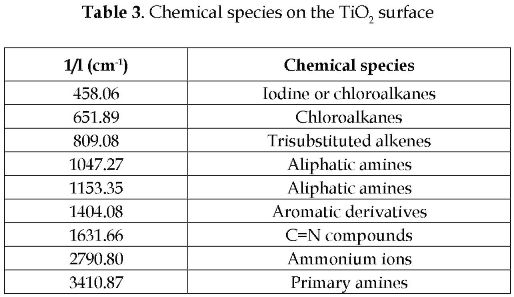
The catalyst lost its capability to degrade the pesticide mixture as shown in Figure 4. In this plot, all the tests were carried out with the same solution (TOC0 = 85 ppm). The TiO2 adsorption capability showed a decrease of 10.92 % after the first reuse. This adsorption capability was calculated from the TOC concentrations measured at the beginning of reuse runs and 20 minutes after (read as negative values on the x-axis). The TOC concentrations increasing trend indicated desorption of cationic species from the catalyst surface. The catalyst color changed from white to brown. This was due to chemical species adsorbed on the catalyst surface. This phenomenon was similar to one already reported in the literature [7]. The chemical species present in the TiO2 surface was identified by IR analysis. The spectrogram is showed in Figure 5, and the chemical species corresponding to the spectrogram peaks are listed in Table 3.
The presence of a large variety of nitrogen compounds in the catalyst surface prevented the adsorption and photodegradation of other chemical species. The loss of activity, represented as the decrease of mineralization capability, was clear from the increase in TOC concentration, and it was due to the desorption of carbonyl compounds.
Photocatalytic treatment of pesticides, such as diuron, and 2,4-D, produces several types of carboxylic acids, which end up being transformed to amines and aliphatic compounds after decarboxylation reactions [5]. The preferences of the hydroxyl free radicals depend on pH directly. For diuron, the oxidation of methyl groups occurs in acidic medium, whereas the hydroxyl radicals attack to aromatic rings decrease [12]. The attack to methyl groups is the responsible of the major production of carboxylic acids. After three reuses, these carboxylic compounds do not adsorb on the TiO2 surface. This is suggested by the chemical diffusion of these species to the slurry bulk; thus, the TOC concentration tended to increase. The presence of halides in the TiO2 surface is due to the chlorine present in chemical structures of the ametryne and the 2,4-D.
CONCLUSIONS
For the conditions studied, the catalyst lost its reuse and adsorption capabilities rapidly. This was due to the initial pH considered for the reuse tests, because it favored the carboxylic intermediates desorption, specially the ones derived from the 2,4-D decomposition. Although the adsorption equilibrium was achieved after 20 minutes of darkness, the pH variation during the photocatalytic process (irradiation period) affected the chemical species adsorption significantly, with the results commented before.
References
[1] X. Doménech, M. Litter, W. Jardim, "Procesos avanzados de oxidación para la eliminación de contaminantes", en Eliminación de contaminantes por Fotocatálisis Heterogénea. M. Blesa, Ed. La Plata (Argentina): CYTED, 2001, pp. 3 - 26. [ Links ]
[2] S. Malato, J. Blanco, A. Vidal, C. Richter, "Photocatalysis with solar energy at a pilot-plant scale: An overview", Applied Catalysis B: Environmental, vol. 37, pp.1-15, 2002. [ Links ]
[3] S. Malato, "Photocatalytic reactors for the treatment of liquid wastewater in the presence of solar irradiation", Presented at 1st seminar of Advanced oxidation methods of the trat ment of liquid an Air Waste, Aristotle University of Thessaloniki, Greece, February, 2004. [ Links ]
[4] J. Herrmann, Ch. Guillard, "Photocatalytic degradation of pesticides in agricultural used waters". Academic Science Paris, vol. Chemistry 3, N.° IIc, pp. 417-422, 2001. [ Links ]
[5] I. Konstantinou, T. Albanis, "Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: intermediates and degradation pathways", Applied Catalysis B: Environmental, vol. 42, pp. 319-335, 2003. [ Links ]
[6] K. Venkata, M. Subrahmanyam, P. Boule, "Immobilized TiO2 photocatalyst during long-term use: decrease of its activit", Applied Catalysis B: Environmental, vol. 49, pp. 239-240, 2004. [ Links ]
[7] J. Araña, E. Pulido-Melián, V. Rodríguez-López, A. Peña-Alonso, J. Doña-Rodríguez, O. Gónzalez-Díaz, et al., "Photocatalytic degradation of phenol and phenolic compounds. Part I: Adsorption and FTIR study". Journal of Hazardous Materials, vol. 143, pp. 520-528, 2001. [ Links ]
[8] P. Pekakis, A. Xekoukoulotakis, D. Mantzavinos. "Treatment of textile dyehouse wastewater by TiO2 photocatalysis", Water Research, vol. 40, pp. 1276-1286, 2006. [ Links ]
[9] J. Blanco, S. Malato, C. Estrada, E. Bandala, S. Gelover, T. Leal, "Purificación de aguas por fotocatálisis heterogénea: Estado del arte", en Eliminación de contaminantes por fotocatálsis heterogénea. M. Blesa, Ed. La Plata (Argentina): CYTED, 2001, pp. 51 - 75. [ Links ]
[10] K. Tanaka, K. Reddy, "Photodegradation of phenoxyacetic acid andcarbamate pesticides on TiO2". Applied CatalysisB Environmental, vol. 39, pp. 305-310, 2002. [ Links ]
[11] R. Candal, S. Bilmes, M. Blesa, "Semiconductores con actividad fotocatalítica", en Eliminación de contaminantes por fotocatálisis heterogénea. M. Blesa, Ed. La Plata (Argentina): CYTED, 2001, pp. 79 - 101. [ Links ]
[12] V. Sarria, S. Parra, N. Adler, P. Péringer, N. Benitez, C. Pulgarín, "Recent developments in the coupling of photoassisted and aerobic biological processes for the treatment of biorecalcitrant compounds". Catalysis Today, vol. 76, pp. 301-315, 2002. [ Links ]