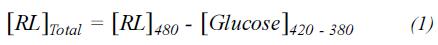Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383On-line version ISSN 2382-4581
C.T.F Cienc. Tecnol. Futuro vol.1 no.3 Bucaramanga Jan./Dec. 1997
A. L. PIMIENTA R1., M. P. DÍAZ M.*1, F. G. CARVAJAL S1. and J. L. GROSSO V1.
1Ecopetrol -Instituto Colombiano del Petróleo, A.A. 4185 Bucaramanga, Santander, Colombia
* To whom all correspondence should be addressed.
ABSTRACT
The biosurfactant production by strains of Pseudomonas aeruginosa isolated from Colombian hydrocarbon contaminated sludges has been determined. The methodology included the isolation of microorganisms, standardization of batch culture conditions for good surfactant production and characterization of the produced rhamnolipid. Several carbon sources were evaluated with regard to the growth and production curves. The stability of the rhamnolipid was also determined under variable conditions of pH, temperature and salt concentration. The strain Pseudomonas aeruginosa BS 3 showed biosurfactant production capabilities of rhamnolipid resulting in concentrations up to 2 g-dm-3 with surface tensions of 30 -32 mN-m-1 in batch cultures with commercial nutrients.
Keywords: biosurfactant, Pseudomonas, rhamnolipid, glycolipid, surface active compound.
RESUMEN
Se determinó la capacidad de producción de biosurfactantes de cepas de Pseudomonas aeruginosa provenientes de lodos contaminados con hidrocarburo de diferentes regiones del país. La metodología utilizada comprendió el aislamiento de microorganismos, estandarización de condiciones de cultivo por lotes para una buena producción de tensoactivo y caracterización del ramnolípido producido. Se hizo énfasis en la evaluación de las fuentes de carbono más eficientes relacionada con las curvas de crecimiento y producción. También se determinó la estabilidad del ramnolípido frente a las variaciones de pH, temperatura y salinidad. La cepa Pseudomonas aeruginosa BS 3 permite producir biosurfactante del tipo ramnolípido con rendimientos hasta de 2 g-dm-3 con tensión superficial de 30-32 mN-m-1 en cultivos por lotes con nutrientes grado comercial.
Palabras claves: biosurfactantes, Pseudomonas, ramnolípido, glicolípidos, la superficie de compuesto activo.
INTRODUCTION
Surfactants are substances widely used in various industrial processes such as pharmaceutic, cosmetic, petroleum, food production and are frequently chemically synthesized. These chemical compounds are not biodegradable and can be toxic to the environment. Biosurfactants, however, have been shown in many cases to have equivalent emulsification properties and are biodegradable. Biosurfactants are surface active compounds mainly produced by microorganisms and synthesized in cells using diverse substrates, being totally biodegradable in aerobic and anaerobic conditions as well as non-toxic (Van Dyke et al., 1993; Kosaric et al., 1993; Banat, 1995; Ducret et al., 1995).
The emulsifying properties of these substances and/ or their derived detergents are due to the existence of hydrophobic and hydrophilic moiety within the same molecule, which allows them to interact between two phases with different physicochemical characteristics. Biosurfactants can be glycolipids, lipopeptides/lipoproteins, lipopolysaccharides, substituted fatty acids and phospholipids according to their radicals (Duvnjak et al., 1982; Montes de Oca, 1992; Fiechter, 1992; Hommel, 1990).
The efficiency and effectiveness of these compounds are determined by their ability to reduce the surface tension of an aqueous solution and by the measurement of the critical micelle concentration (CMC) or concentration of biosurfactant necessary to achieve the lowest possible surface tension (Fiechter, 1992).
Rhamnolipids are normally synthesized by microorganisms to facilitate the uptaking of an insoluble substrate. For example, when oil is present in contaminated areas, the native microorganisms are selected for their assimilation capacity causing the availability and incorporation of the substrate to the cell for posterior metabolism (biodegradation) (Hommel, 1990; Zhang and Miller, 1994; Volkering et al., 1995)
There are many different microorganisms capable of synthesizing biosurfactants, Pseudomonas fluorescens, P. aeruginosa, are some of them (Kosaric et al., 1993; Hommel, 1990; Fiechter, 1992). Although extensively investigated and with a promising future, glycolipids have not been commercialized due to then high prize. Rhamnolipids, produced by Pseudomonas species are a kind of glycolipids (Hisatsuka et al., 1971; Hauser and Karnovsky, 1954).
Due to their biological origin and functional properties, biosurfactants can be used in various petroleum industrial processes, including emulsification and deemulsification, separation, formation of low viscosity production of emulsions to transport heavy crudes, emulsion washing, formation of slurries, corrosion inhibition, enhance oil recovery, hydrocarbon biodegredation promotion (Kosaric et al., 1983; Díaz, 1991). If economically competitive, biosurfactants have the potential to replace synthetic surfactants, as they possess similar structural and physical properties, and are produced by renewable substrates, with the advantage of being degradable. When analyzing their economic viability, it is important to consider the nutritional requirements of the microorganism, the cost of the carbon sources employed (in bioremediation, a contaminating substance can be used as the substrate), the culture conditions and the design of a simple procedure to separate the product.
The aim of the present investigation was to select and evaluate the biosurfactant production capacity of native strains of Pseudomonas aeruginosa at laboratory level, with possible application in the petroleum industry.
EXPERIMENTAL METHODOLOGY
Microorganisms
Strains of Pseudomonas aeruginosa obtained by conventional methods (direct isolations on cetrimide and nutrient agar plates, and dilutions and growth in enrichment saline media), from sludges and waters taken from hydrocarbon contaminated areas in zones adjacent to petrochemical industries or crude oil spills.
The isolated bacteria were identified with the BIOLOGTM system for Gram negative bacteria; 95 tests were conducted to evaluate the carbon sources. The results were compared with the Microlog software database while the determination of the coefficient of similarity was used as the confidence criteria of the identification (Biolog, 1993).
The bacteria were frozen with glycerol at -193 K (fresh axenic culture in saline production medium: 20% V/V glycerol solution, in ratio 1:1) and fresh cultures on cetrimide agar plates (Merck, Darmstadt).
The selection and adaptation of the bacteria was carried out by both quantitative tests of the production capacity of rhamnolipid in saline media, and qualitative tests for foam production and reproducibility of results.
Culture Media
The medium used for the growth and production of biosurfactants is a variation of the one reported by Hauser and Karnovsky (1954), using a 1% carbon source. It was identified as a Saline Production Medium (SPM) (all the components are per dm-3): K2HPO4, 7 g; KH2PO4, 3 g; MgSO4 7H2O, 0.1 g; (NH4)2SO4, 1 g; glycerol, 10 cm3 (Merck Darmstadt); pH 7,0. These chemicals were changed from analytical grades to commercial grades. Rhamnolipids are formed by P. aeruginosa grown on different carbon sources such as glycerol, glucose, vegetal oil, molasses and n-paraffin, etc., so as to evaluate and select an appropriate culture media (Guerra-Santos et al., 1984; Mercadé et al., 1993; Robert et al., 1991; Ramana and Karanth, 1989; Van Dyke et al., 1993; Zhang and Miller, 1994; Ghurye et al., 1994).
Nutrient and cetrimide agar plates were used to monitor and count the bacteria population.
Standarization of the culture conditions and saline composition
Different culture media were selected and evaluated with isolated strains in axenic cultures. The selection criteria was that more than 50% of the strains had grown, formed foam and produced a minimum of 500 mgdm-3 of rhamnolipid. A 100 -200 rpm range of agitation and 5,0 -9,0 range pH were selected. (Van Dyke et al., 1993; Ramana and Karanth, 1989; Ghurye et al., 1994). Progressive microorganism adaptation processes by successive transferences to obtain strains that produce high level of rhamnolipid in less time were conducted. At the same time, the kinetic evaluations of the microorganisms were completed, which also favored the reduction of the process time.
The bacteria inocula was prepared by addition of cells from the solid medium to 5 cm3 of SPM followed by incubation at 305 K (32 °C) and rotatory agitation at 150 rpm. After 24 hours, this culture was transferred to another 50 cm3 of sterile medium, which was incubated under the same conditions for approximately 7 to 10 days. A saline medium without inoculation using glycerol or the corresponding carbon source was employed as a negative control.
Evaluation of the carbon sources
Glycerol, sucrose, molasses, glucose and vegetal oil were evaluated in order to determine the most efficient carbon source. These sources were supplemented at 1% in SPM with analytical and commercial grade chemicals, and then evaluated following the general procedure.
Determination of rhamnolipid
The analytical evaluations for rhamnolipid were carried out by the phenol-sulfuric acid method, determining rhamnose in a glycerol and vegetable oil media and by the L-cystheine-sulfuric acid method for other sources of carbon containing glucose (Whistler and Wolfrom, 1962). Pretreatment of the samples was done by removing biomass by centrifugation at 6.000 rpm for 15 minutes. All measurements were carriet out in a UV-VIS Cary Varian 1E Spectrophotometer.
Equation 1 was used for the calculation of the concentration of rhamnose. These values were converted to rhamnolipid units.
Measurement of surface tension and CMC determination
The surface tension was measured by the maximum bubble pressure method using a Sensadyne 6.000 tensiometer. The determination of the Critical Micelle Concentration (CMC) was conducted by conventional methods (Margaritis et al., 1979), using the rhamnolipid produced from glycerol as a carbon source in increasing concentrations of 50, 100, 200, 300, 500, 800, 1600 and 2.000 mg.dm-3, and the measurement of the associated surface tension.
Evaluation of the growth and production of rhamnolipid by Pseudomonas aeruginosa
These evaluations were achieved by performing growth and production experiments according to the culture conditions described above separately in 50 cm3 flasks containing SPM with commercial grade chemicals and glycerol and vegetable oil as carbon sources.
Evaluating Biosurfactant Stability
For comparison purposes, the surfactants used were Triton X-100 and LAS (linear alquilbenzene sulfonate) analytical and commercial grades. Triton X-100 is a nonionic surfactant and LAS analytical grade is linear anionic surfactant. The biosurfactant was produced according to the culture conditions described above. The biomass was removed by centrifugation and the level of rhamnolipid was determined. Stability was evaluated by measuring the surface tension. The sole indirect criteria applied to determine tenso-active activity was the measurement of the tension surface (Margaritis et al., 1979).
Assessment of the temperature resistance was carried out at 297, 313, 333 and 353 K. This involved exposing the product at each temperature for periods of 0,5 to 1 hour. The pH stability tests were performed by adding diluted sulfuric acid or sodium hydroxide so that solutions of pH 2 to 12 were obtained. Salt was added to the solutions in increasing sodium chloride concentrations (0, 1, 5, 10 and 20% (w/v)) in order to establish stability.
Evaluation of emulsifying properties
To determine the emulsifying properties of the biosurfactant, inverse O/W emulsions were prepared with heavy crude oil, varying the concentration of the surface-active ingredient.
RESULTS AND DISCUSSION
Evaluation of different native strains of Pseudomonas
Pseudomonas was selected for its nutritional and biochemical versatility as well as for the simplicity of the culture conditions; the latter being the main reason for their wide presence in different habitats (Krieg and Holt, 1984). Among the strains recovered from hydrocarbon contaminated sludges and waters, those that corresponded to the morphology of Pseudomonas were individually studied. The morphologic characteristics of these strains and the biosurfactant production results are shown in Table 1.
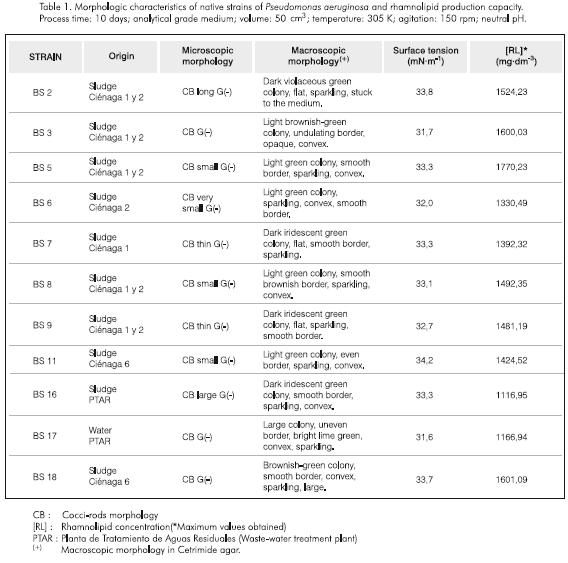
The time of appearance, the amount of foam generated, raising viscosity and darkering of the initial color through time were used as qualitative measures of the presence of the rhamnolipid. All the identified strains belong to the same species, Pseudomonas aeruginosa, but each microorganism had different morphological characteristics.
Taking into account the nutritional and activation characteristics for the kinetic study, a particular strain was considered rather than a group of microorganisms. Although the Pseudomonas aeruginosa BS 5 showed in some cases higher levels of production, the Pseudomonas aeruginosa BS 3 was selected for its production stability and reproducibility properties.
Influence of culture conditions on rhamnolipid production rates
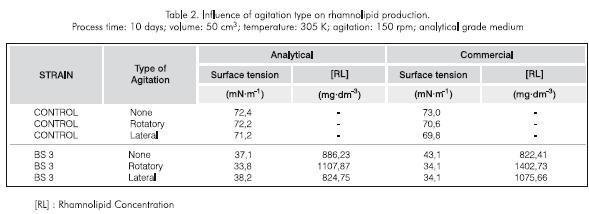
Agitation. Repeated evaluation trials indicated that agitation did indeed influence production, and that the culture without agitation produced less rhamnolipid than with any of the agitation systems. The system without agitation does not present a good time-oxygen transfer which increases the process time for an efficient production. Additionally, the type of agitation employed was an important factor in the biosurfactant production. A higher concentration of rhamnolipid was achieved by rotatory shaking (orbital agitation) when compared to the thermoregulated bath (lateral agitation) and the control without agitation. The degree of homogenization of the culture achieved during agitation marked the difference between the two agitation systems (Table 2).
pH Value. The minimum surface tension and highest recovery of rhamnolipid was obtained with pH between 7,0 and 8,0 (Table 3). Besides, in all cases of the fermentation process, the pH decrease included alkaline pH. For this reason, it was decided to work at a neutral pH and thus eliminate the use of alkali and reduce process costs.
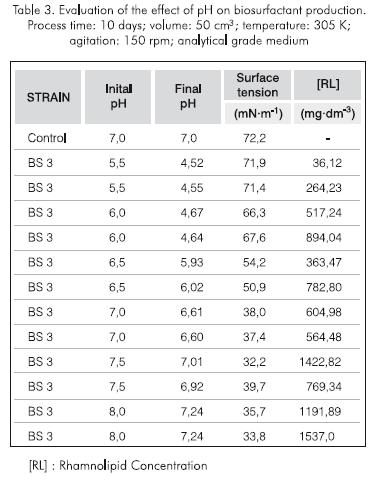
It was noticed that the pH value induced strong fluctuations in the production of rhamnolipid, probably due to the kinetic and metabolic behavior of the microorganism, which produces acids from carbohydrates as it grows thus decreasing the pH (Krieg and Holt, 1984). This condition affects the microorganism in distinct ways, one of which is the deactivation of its enzymes if the pH value is outside their normal range of operation. This effect is not drastic as alkaline pH values (Bohinski, 1991).
It is important to mention that the cultures were not maintained at a constant pH because it changed freely.
Saline purity of chemicals. Initially, the cultures in analytical grade media exhibited higher production of surfactant than those performed in commercial grade media, suggesting that the purity of the components influences the output rate of the bacteria. However, as the bacteria progressively adapted to these components this performance criteria was inverted. This is possibly attributed to the presence of trace impurities or ions found in running water, which served as micronutrients for the growth and production of rhamnolipid.
Process time. The standardization of the culture conditions involved the progressive adaptation of the microorganisms to the culture medium. This was achieved by successive feeding steps with metabolically active seed cultures from previous experiments. As a result, the initial process time of 15 days was progressively reduced to 10 and 7 days.
Evaluation of carbon sources. The following carbon sources were evaluated: glycerol, molasses, sucrose, glucose and vegetal oil.
The metabolic behavior of the BS 3 strain varied considerably with the different carbon sources. The general trend was an improvement in rhamnolipid production and a reduction in surface tension using commercial grade media. It is interesting to note, that although there were numerous interferences in the analytical method (other carbohidrates) to determine the rhamnolipid produced with sucrose and molasses, the surface tension results indicated that there was no biosurfactant production.
These interferences were due to the availability of more than one carbon substrate for the bacteria. As the analytical method included indirect determination of rhamnose concentrations (aldohexose), the nondegraded substrate caused interferences in the colorimetric method. This difficulty was overcame by carrying out evaluations at different wave lengths, which corrected the absorbance of the interferent.
Glycerol was observed to give the highest rates of production of the different soluble carbon sources studied. The production with vegetal oil was either similar or higher than the one obtained with the soluble sources. This implies that a selective pressure effect is acting on the microorganism, to produce the biosurfactant and thus makes the substrate available for metabolism (Mercadé et al., 1993, Zhang and Miller, 1995). Table 4 shows the test results for the various carbon sources studied.
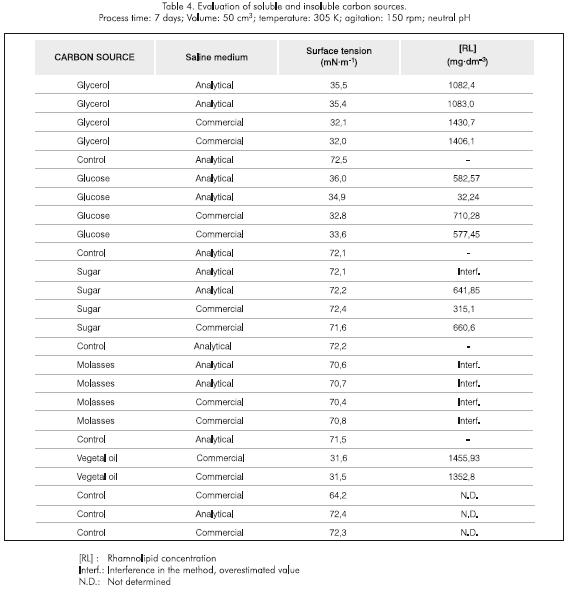
Determination of CMC of the rhamnolipid
The Critical Micelle Concentration of the rhamnolipid was determined in crude extracts (without purification), using glycerol as the carbon source. However, it should be noted that the carbon source is in fact irrelevant, since increasing concentrations of rhamnolipid are considered. The CMC of the rhamnolipid produced by Pseudomonas aeruginosa BS 3 was found to be in the 300 -400 mgdm-3 range. These results are given in Figure 1.
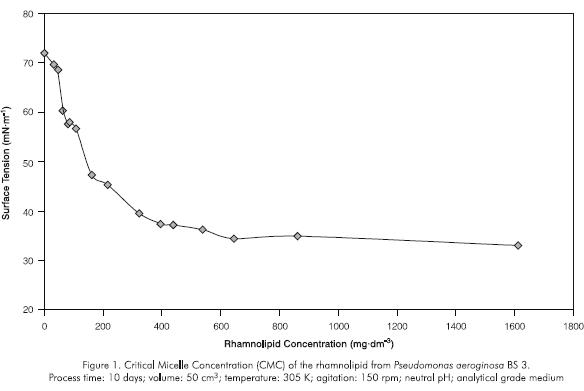
Evaluation of the growth and production of the rhamnolipid by Pseudomonas aeruginosa
Growth and Production Curves. Growth and production curves using glycerol and vegetal oil in commercial grade media were used to compare the production of soluble and insoluble carbon sources.
During the first 30 hours of evaluation, the glycerol growth curve exhibited the latent and exponential phases (Figures 2 and 3). Discontinuity in the different parameters was observed in the following 18 hours (stationary phase) for all variables except for the absorbance (Figures 2, 3 and 4). Such phenomena can be associated with the formation of non determined byproducts of the bacterial metabolism which act as multiple carbon sources, producing a diauxic behavior. Moreover, during the stationary phase metabolic activity decreases, there is competition for the residual substrate and culture saturation occurs due to toxins and non metabolizable byproducts accumulation which can cause deviations in bacterial metabolism.
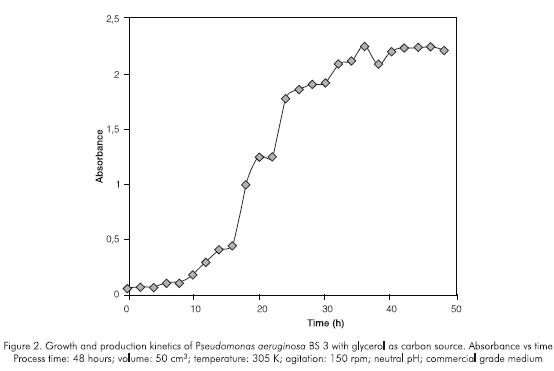
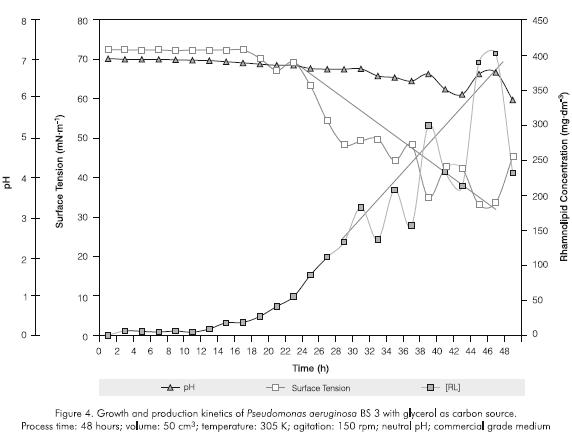
Surface tension was reduced by increasing the rhamnolipid concentration in the culture medium. The curve stabled off at a rhamnolipid concentration of 300 to 400 mg.dm-3 (CMC) after about 48 hours. The timerhamnolipid concentration relationship indicates that biosurfactant production increased at around the 20 hours point (Figure 4). Although the rhamnolipid appears at the same time with the microbial growth, its accelerated production is present in the stationary phase. For this reason, the product formation appeared to be partly growth-associated as described by other authors (Persson et al., 1988; Guerra-Santos et al., 1986). Moreover, the influence of limited concentration or of the source of inorganic nutrients such as nitrogen, iron, phosphorous on growth and biosurfactant production could be presented but not evaluated (Guerra-Santos et al., 1984; Guerra-Santos et al., 1986; Persson et al., 1988; Mulligan and Gibbs, 1989; Mulligan et al., 1989; Ochsner et al., 1995).
Figure 3 and 5 presents the microbial count, pH, surface tension and rhamnolipid concentration parameters produced using a commercial vegetal oil. The behavior was similar to the one observed with glycerol but more stable. The pH steadily decreased; rhamnolipid production started earlier (after 10 -12 hours) which reflected in a reduction of surface tension and a slight increase of the microbial growth. This fact is characteristic of insoluble substrates which make a selective pressure in the first steps of the rhamnolipid production.
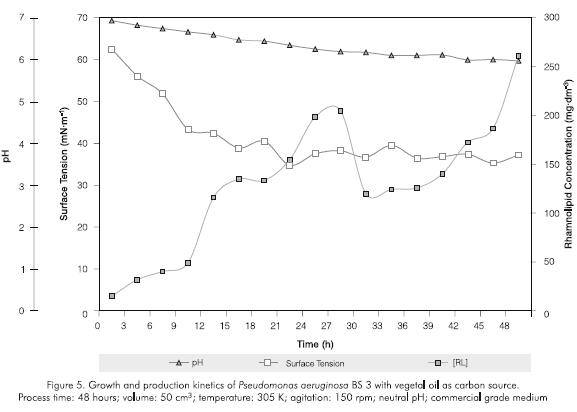
A drop was observed in the amount of rhamnolipid produced which is probably related to the reduction and stabilization of the microbial count in the stationary phase
Comparing the two carbon sources used, higher levels of rhamnolipid were produced using glycerol in stationary phase at 46 hours.
Evaluation of biosurfactant stability
The biosurfactant exhibited good tenso-active properties between a pH of 5 and 10 (Figure 6); resisted temperatures up to 353 K during one hour (Figure 7); and salt concentrations of up to 5% (Figure 8). A permanent surface tension of the aqueous solution in the 30 -45 mNm-1 range was used as a qualitative measure of stability. The biosurfactant rapidly loses this property after this limit. At higher values of 45 mNm-1, the surfactant losses its capacity to interact between two phases with different physicochemical characteristics (Kosaric, 1993).
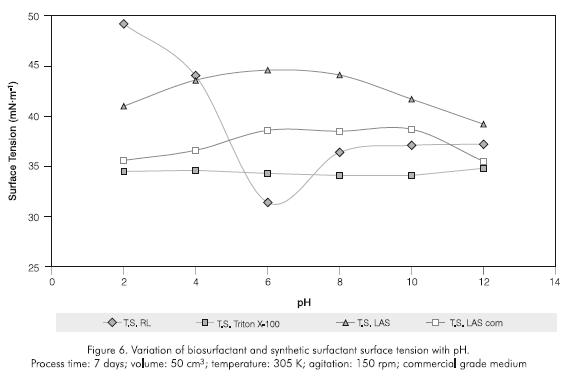
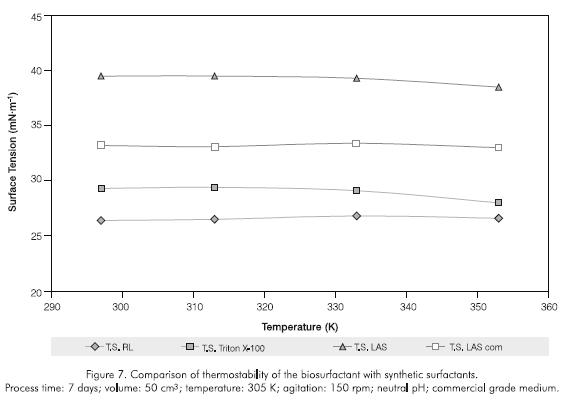
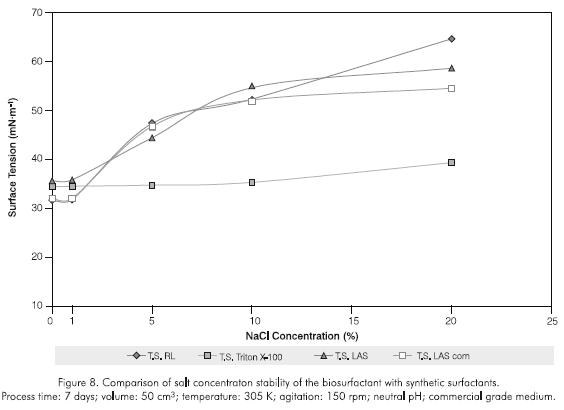
The rhamnolipid showed greater temperature stability than the other surfactants studied, with tensions not exceeding 32 mNm-1. The evaluation time was set taking into account variables such as costs, efficiency in real washing emulsion processes and operation speed in biodegradation systems, which must be completed in a similar period of time. With regards to salt concentration, only Triton X-100 was stable during the whole evaluation (34,5 -39,3 mN.m-1), while LAS analytical and commercial grades were equivalent or less effective than the rhamnolipid. Triton X-100 showed the greatest stability to pH (surface tensions 34,1 -34,8 mN.m-1), LAS gave tensions exceeding 38 mN.m-1, while the biosurfactant was more effective than the previously mentioned in the 5,5 -7,5 pH range.
Evaluation of the biosurfactant emulsifying properties
Inverse low viscosity emulsions were prepared with the biosurfactant and Castilla heavy crude oil, using an active ingredient concentration of 500 mg.dm-3 or greater (with respect to the total emulsion volume). Inverse emulsions O/W 50/50 with 40 cP viscosity and 30 micron particle size were obtained. In addition, stable inverse emulsions O/W 60/40 were formed using active ingredient concentrations of 800 mg.dm-3 or greater.
CONCLUSIONS
-
Strains of Pseudomonas aeruginosa isolated from Colombian hydrocarbon contaminated sludges have the ability to produce surface active compounds. Pseudomonas aeruginosa BS 3 was selected as the most efficient strain in the evaluation.
-
A methodology for producing high output levels of biosurfactant by batch cultures of Pseudomonas aeruginosa was determined.This involved controlling agitation, temperature, pH and salt concentration conditions. Media employing analytical grade chemicals were successfully replaced by commercial grade media. The emulsifying property of the biosurfactant was also verified with heavy crude oil.
-
Soluble carbon sources were found to be the most effective ones for producing biosurfactants. However, insoluble carbon source acts as a limiting agent, such that the assimilation of the carbon source reduces biosurfactant production time.
-
The rhamnolipid produced by Pseudomonas aeruginosa BS 3 is a partly growth-associated metabolite that starts its production in the first 24 hours of incubation. Production starts after aproximatelly 20 hours for soluble carbon sources and after 10 to 12 hours for the insoluble ones.
-
The rhamnolipid produced by the bacteria is an efficient and effective tensoactive agent showing surface tension reductions up to 30 -32 mN.m-1 for aqueous solutions.
-
The Critical Micelle Concentration of the rhamnolipid was found to be in the 300 -400 mg.dm-3 range (biosurfactant without purification). This nonpurified condition makes it very economical for industrial production processes.
-
Compared with other synthetic surfactants, the biosurfactant was found to be very stable under variable pH, temperature and salt concentration test conditions.
-
More work remains to be done in order to evaluate and optimize the production and recovery of biosurfactants and their stability and emulsifying properties. It will also be important to conduct the chemical characterization of the molecules produced by Pseudomonas aeruginosa BS 3.
ACKNOWLEDGMENTS
The authors express their thanks to Amleto León, Gladys Rosero, Luis Ortiz, María Carolina Vargas and Esperanza Torres for their valuable collaboration in completing this work.
REFERENCES
Banat, I. M., 1995. "Biosurfacts production and possible uses in microbial enhanced oil recovery and oil pollution remediation: A review", Bioresource Technology, 51: 1-12. [ Links ]
Biolog, Inc., 1993. MicroStation™ System Release, Versión 3.50, USA. [ Links ]
Bohinski, R. C, 1991. Bioquímica, Editorial Addison-Wesley Iberoamericana SA, 5a Edición. USA. [ Links ]
Díaz, M. P., 1991. "Estudio preliminar de la aplicación de biosurfactantes en el manejo de crudos pesados". Tesis de Maestría, UIS. [ Links ]
Ducret, A., Giroux, A., Trani, M. and Lortie, R, 1995. "Enzymatic preparation of biosurfactants from sugars or sugar alcohols and fatty acids in organic media under reduced pressure", Biotechnology and Bioengineering. 48 (3): 214-221. [ Links ]
Duvnjak, Z., Cooper, D. G and Kosaric, N., 1982. "Production of surfactant by Arthrobacter paraffineus PSCC 19558", Biotechnol. Bioeng, 24: 165-175. [ Links ]
Fiechter, A., 1992. "A review. Biosurfactants: moving towards industrial application", Tibtech, 10: 208 -217. [ Links ]
Ghurye, G L., Vipulanandan, C. and Willson, R C, 1994. "A practical approach to biosurfactant production using nonaseptic fermentation of mixed cultures", Biotechnology and Bioengineering, 44 (5): 661 -666. [ Links ]
Guerra-Santos, L., Kãppeli, O. and Fiechter, A., 1984. "Pseudomonas aeruginosa biosurfactant production in continuous culture with glucose as carbon source", Applied and Environmental Microbiology, 48 (2): 301-305. [ Links ]
Guerra-Santos, L., Kãppeli, O. and Fiechter, A., 1986. "Dependence of Pseudomonas aeruginosa continuous culture biosurfactant production on nutritional and environmental factors", Applied and Environmental Microbiolog., 24: 443-448. [ Links ]
Hauser, G. and Karnovsky, M., 1954. "Studies on the production of glycolipids by Pseudomonas aeruginosa", Biotech, 68: 645 -654. [ Links ]
Hisatsuka, K.; Nakahara, T.; Sano, N. and Yamada, K., 1971. "Formation of rhamnolipidby Pseudomonas aeruginosa and its function in hydrocarbon fermentation", Agrie. Biol. Chem., 35 (5): 686 -692. [ Links ]
Hommel, R., 1990. "Formation and physiological role of biosurfactants produced by hydrocarbon utilizing microorganisms", Biodegradation, 1: 107-119. [ Links ]
Kosaric, N., Gray, N.C.C. and Cairns, W.L., 1983. "Microbial emulsifiers and de-emulsifiers", in Rehm H-J & Reed G. (Eds.), Biotechnology, Verlag Chemie, Weinheim, 3: 575 -592. [ Links ]
Kosaric, N., Cairns, W. L. and Gray, N.C.C. (Eds.), 1993. "Surfactant science series", Biosurfactants and biotechnology, Marcel Dekker, Mew York Basel, 25. [ Links ]
Krieg, N. and Holt, J (Eds.), 1984. Bergey's manual of systematic bacteriology, Editorial Board, Baltimore, USA, voll. [ Links ]
Margaritis, A., Zajic, J. and Gerson, D.,1979. "Production and surface active properties of microbial surfactants", Biotechnology and Bioengineering, 21: 1151-1162. [ Links ]
Mercadé, M. E., Manresa, M. A., Robert, M., Espuny, M. J., de Andrés, C. and Guinea, J., 1993. "Olive oil mill effluent (OOME). New substrate for biosurfactant production", Bioresource Technology, 43: 1-6. [ Links ]
Montes de Oca G, M. A., 1992. "Producción de biosurfactantes microbianos", Revista del Instituto Mexicano del Petróleo, 24 (4): 68 -78. [ Links ]
Mulligan, C. and Gibbs, B. E, 1989. "Correlation of nitrogen metabolism with biosurfactant production by Pseudomonas aeruginosa", Applied and Environmental Microbiology, 55 (11): 3016-3019. [ Links ]
Mulligan, C, Mahmourides, G and Gibbs, B. F, 1989. "The influence of phosphate metabolism on biosurfactant production by Pseudomonas aeruginosa", Journal of Biotechnology, 12: 199-210. [ Links ]
Ochsner, Urs A., Hembach, T. and Fiechter, A., 1995. "Production of Rhamnolipid Biosurfactants", Advances in Biochemical Engineering/ Biotechnology, Springer Verlag Berlin Heidelberg, 5 3. [ Links ]
Persson, A., Osterberg, E. and Dostalek, M., 1988. "Biosurfactant production by Pseudomonas fluorescens 378: Growth and product characteristics", Applied Microbiology and Biotechnology, 29: 1-4. [ Links ]
Ramana, K. V. and Karanth, N. G, 1989. "Production of biosurfactants by the resting cells of Pseudomonas aeruginosa CFTR-6"', Biotechnology Letters, 11 (6): 437 -442. [ Links ]
Reiling, H. E., Thanei-Wyss, U., Guerra-Santos, L. H., Hirt, R. Kãpelli, O. and Fiechter, A., 1986. "Pilot plant production of rhamnolipid biosurfactant by Pseudomonas aeruginosa", Applied and Environmental Microbiology, 51(5): 985-989. [ Links ]
Robert, M., Mercadé, M. E., de Andrés, C, Espuny, M. J., Manresa, M. A. and Guinea, J., 1991. "Optimización de la producción de biotensoactivos por Pseudomonas aeruginosa 44T1", Fase. 1, 42: 1-7. [ Links ]
Van Dyke, M. I., Couture, P., Brauer, M., Lee, H. and Trevors, J.T., 1993. "Pseudomonas aeruginosa\JG2 rhamnolipid biosurfactants: structural caracterization and their use in removing hydrophobic compounds from soil". Can. J. Microbiol, 39: 1.071 -1.078. [ Links ]
Volkering, F, Breure, A. M., Van Andel, J. G and Rulkens, W. H., 1995. "Influence of nonionic surfactants on bioavailability and biodegradation of polycyclic aromatic hydrocarbons", Applied and Environmental Microbiology, 61 (5): 1.699 -1.705. [ Links ]
Whistler, R. and Wolfrom, M., 1962. Methods in carbohydrates chemistry, Academic Press, New York. [ Links ]
Zhang, Y and Miller, R. M., 1994. "Effect of a Pseudomonas rhamnolipid biosurfactant on cell hydrophobicity and biodegradation of octadecane". Applied and Environmental Microbiology, 60 (6): 2.101 -2.106. [ Links ]
Zhang, Y. and Miller, R. M., 1995. "Effect of rhamnolipid (biosurfactant) structure on solubilization and biodegradation of n-alkanes", Applied and Environmental Microbiology, 61 (6): 2.247-2.251. [ Links ]