Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383On-line version ISSN 2382-4581
C.T.F Cienc. Tecnol. Futuro vol.1 no.4 Bucaramanga Jan./Dec. 1998
DIESEL CHARACTERIZATION BY HIGH RESOLUTION MASS SPECTROMETRY-GAS CHROMATOGRAPHY
ABSTRACTT
High-resolution mass spectrometry-gas chromatography is combined with the HC22 method in order to obtain a detailed information about the chemical composition of diesel and the distribution of different compound types in terms of its final boiling temperature from a single analysis. The total time elapsed from sample injection and signal processing to obtain final results is 90 minutes. This fact makes this methodology a new and very important tool for the decision making process concerning the most suitable final boiling temperature and the type of treatment of the product in order to obtain diesel that fulfills the international standards. The consistency and repeatability of the experimental results are demonstrated.
Keywords: hydrocarbon group type analysis, high resolution mass spectrometry, diesel.
RESUMEN
Se combina la espectrometría de masas de alta resolución acoplada a cromatografía de gases con el método HC22 para obtener información detallada acerca de la composición química del diesel y de la distribución de diferentes tipos de compuestos en función de su temperatura final de ebullición a partir de un solo análisis. El tiempo total transcurrido desde la inyección de la muestra y el procesamiento de la señal hasta la obtención de resultados finales es de 90 minutos. Esto hace de esta metodología una nueva y muy importante herramienta para la toma de decisiones a nivel de proceso sobre la temperatura final de ebullición y el tipo de tratamiento más adecuado para obtener un diesel que cumpla con los estándares internacionales. Se demuestra la consistencia y repetibilidad de los resultados analíticos.
Palabras clave: grupo hidrocarburo tipo de análisis. espectrometría de masas alta resolución, diesel.
INTRODUCTION
Nowadays, the crude refining industry is confronted to very strict environmental restrictions. The survival of refineries requires very large investments in order to implement operational modifications to improve the quality of their products. As an example, in the United States the sulfur content has decreased in 80% (from 0,25% to 0,05% in weight) since 1993 and the cetane index was set at 40 as to maintain the aromatic level between 31% and 34%. Further environmental regulations require the total aromatic content to be under 20%. In Colombia, the maximum sulfur content is 0,6% and the minimum acceptable cetane index is 45. The maximum limit hasn't yet been established for aromatic compounds.
Due to the increasing demand of high quality diesel, refiners are utilizing the hydrotreatment option in order to fulfill the environmental regulations for the near future.
The need to count on complete data concerning the chemical composition of the diesel is increasingly important in order to establish correlations between the compounds and their behavior during combustion (Ullman, et al., 1990). On the other hand, the trend to use narrower diesel cuts to fulfill environmental regulations imposes the use of versatile and reliable analytical methodologies to obtain detailed information about the chemical composition of the diesel and the distribution of components in terms of their boiling point.
The hydrocarbon type analysis concept that expresses results in terms of families of components is applied for diesel characterization due to the impossibility of developing a chromatographic system for the separation of diesel components as can be done for gasoline. Under the light of this concept are expressed the results obtained by methods such as the ASTMD1319 to evaluate aromatics, chromatographic methods (Sink, et al., 1994), column chromatography (ASTM D2549) and low resolution mass spectrometry (ASTM D2425) which can be applied to this type of oil fractions.
The analysis of cuts using the above mentioned techniques supplies valuable information on the distribution of families in terms of the boiling temperature; however, the need to previously fractionate the sample constitutes a problem. Moreover, if we also consider the subsequent requirement of component separation by column chromatography for the standard hydrocarbon type analysis methodologies of the cuts by low resolution mass spectrometry, we can clearly see the experimental difficulty encountered by the analytical chemist in obtaining quantitative information concerning the distribution of components in terms of the final boiling point of diesel.
It has been demonstrated that it is possible to obviate the previous component separation step to determine the chemical composition by hydrocarbon type of the diesel or of its cuts by low-resolution mass spectrometry using a purely mathematical procedure for the analysis of the spectral signal (Robinson, 1971). Although this procedure hasn't yet been accepted as a standard methodology, Robinson's method modified by some researchers, has been used on research studies (Wilson et al., 1986).
Today, high resolution mass spectrometry is perhaps the most powerful tool available to chemically characterize oil fractions heavier than gasoline. Through its application, it is possible to obtain complete information about the different hydrocarbon types present in oil fractions (paraffins, cyclo-paraffins, monoaromatics, diaromatics, triaromatics, tetraaromatics and sulfur aromatics) without previous separation of the sample. The high-resolution conditions eliminate the superposition of signals that reduce the accuracy in low-resolution methods, but like these, high resolution methods have not been accepted as standard procedures.
Although this type of analyses was developed during the 60's and 70's (Gallegos et al., 1967; Fisher, 1974) its use wasn't generalized due to the need of sophisticated equipment, personnel and software specialized for this type of analyses as well as to the lack of analytical standards that allowed determining the accuracy of the data obtained through mathematical matrices established in these researches.
The hydrocarbon type analysis by high resolution as it was developed is performed on a representative portion of the mixture with no previous separation. The sample is completely evaporated in a glass system with temperature control (Aghis system). Despite the fact that the previous procedure is very quick, only an average spectrum of the total sample is obtained.
This research evaluates the possibility of combining a gas chromatograph provided with a capillary column acting as a micro distillation unit and the high-resolution mass spectrometer as a detector capable of separating signals corresponding to the different compounds. If it can be demonstrated that the averaged results of the hydrocarbon type analysis obtained through this system are equivalent to the ones obtained through the traditional injection methodology, the chromatographic signal can be processed to obtain the composition of the diesel and the component distribution in terms of the final boiling point through a single analysis.
EXPERIMENTAL PHASE
A refinery diesel sample was selected for this research. The total sulfur content was determined by X-ray fluorescence through the ASTM D4294 method.
The analysis of the sample by mass spectrometry was carried out in a double focusing VG Fisons Autospec Ultima Model coupled to a CE8000 chromatography. A perfluokerosene pressure (PFK) was maintained during the analysis through a hot septum at a constant temperature of 523 K. The magnet was programmed to sweep the 500 - 50 mass interval.
Two different methods were used for the introduction of the sample. The first one was accomplished through an all-glass heated inlet system (Aghis) maintained at 473 K in which the sample completely evaporates allowing the diffusion of a representative part of it into the source through a glass capillary. In the second one, the injection was done through a gas chromatograph (gc) with a 30 meters long 5% phenyl methyl silicone capillary column to separate the components that arrive at the ion source of the spectrometer. The oven temperature was programmed from 333 K to 593 K at an increasing rate of 6 K per minute.
In both experiments the ion source was operated at the same conditions: 523 K, static resolution 3.500 (10% valley), electron energy 70 eV. The sweep rate of the magnet was set at 1 sec/decade.
The mass spectra were calibrated and the PFK peaks removed to make an average spectra of the complete run. When the injection was done trough the Aghis, the average was obtained on the scans located at the top of the signal. When the gas chromatograph was used to inject the sample, all the scans that conformed the ion chromatogram were averaged in their absolute intensity. The averaged spectrum thus obtained was used to calculate composition by the HC22 method from PCMASPEC.
RESULTS AND DISCUSSION
Verification of the applicability of the GC-MS analyses
The signal that arrives at the detector in the analysis of the effluents of a chromatographic column by mass spectrometry is time-variable but it is almost constant when the traditional injection system (Aghis system) is used. Under the light of this factor, it can be easily understood that the accuracy of the results obtained through the traditional system are superior to those obtained by averaging the time-variable chromatographic signal.
Table 1 compares the results between the aghis and the gc sample injection. The first column supplies the results of averaging the scans located at the top of the signal when the Aghis was used as the injection method. The second one gives the resultant data of averaging six entire gc-ms runs. Data seem to indicate that in spite of the fact that there are noticeable differences among components such as tetracycloparafins, penta cycloparafins, triaromatics and dibenzotiophenes which are present in very low concentrations, the analyses are in good agreement with each other.
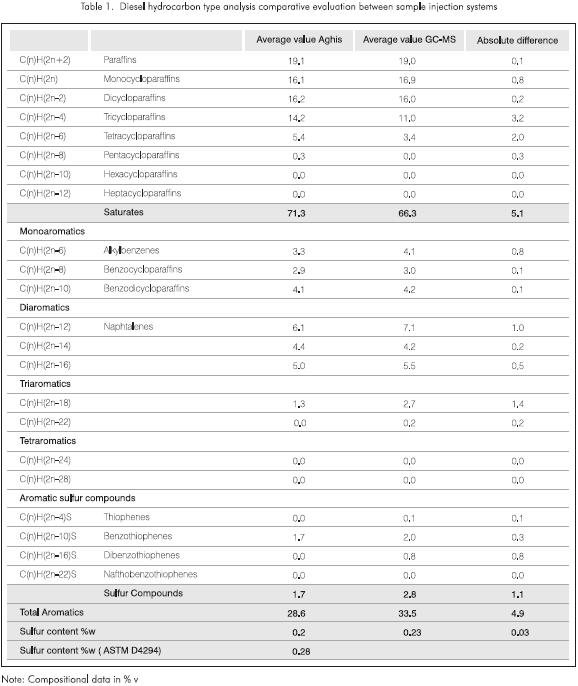
In both cases, the quantity of condensed cycloparaffins is greater than the amount of monocyclopparaffins, an unlogical result considering the molecular weight of this type of sample. This could be related to poor resolution, poor mass assignment and some limitations of the HC22 method and will be evaluated in a subsequent research.
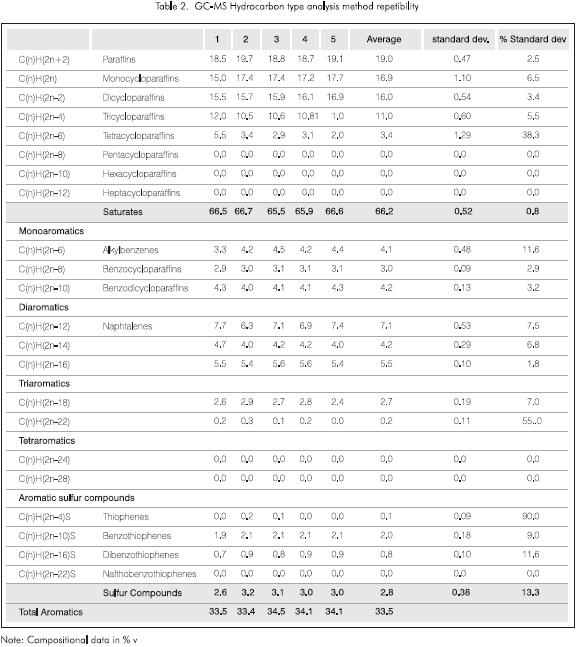
Repeatability of the method
The repeatability of the method was obtained averaging the spectra from six (6) different gc runs of the same sample. The results are shown in Table 2. The maximum standard relative deviation obtained over the components present in a proportion above 1% (v) is 38,3% while a 90% difference was obtained for the thiophenes that are present in a concentration below 1%. Nevertheless, the global repeatability is satisfactory since the high repeatability of the magnet sweep as well as of the chromatographic conditions is known. It is very possible that better results will be obtained with the improvement of the sweep conditions.
Component distribution in terms of the boiling point.
The total ion current chromatogram shown in Figure 1 was taken in order to illustrate how to estimate the distribution of components in terms of the final temperature of the cut. The analysis was conducted from carbon number fourteen (C14) neglecting the signal from the lighter components since they aren't considered representative of this type of product. The figure identifies the n-paraffins for which the normal boiling points are known.
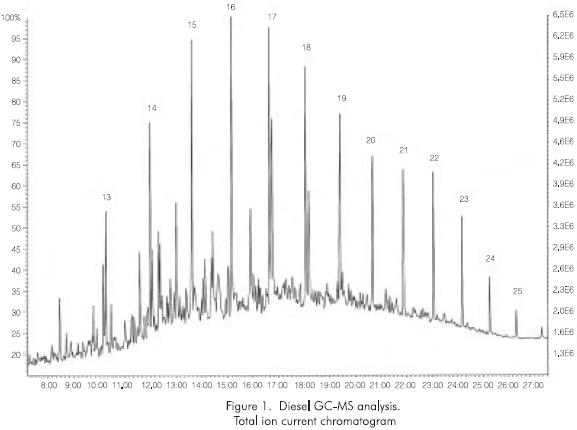
The high resolution spectra from slightly above the C13 peak to and including the C14 peak was averaged, continuing from just above the C14 peak to just above the C15 peak and so forth. Each sum was labeled by the paraffin peak carbon number. The chemical composition by hydrocarbon type was calculated for each averaged spectrum.
The corresponding data are shown in Table 3 together with the intensity values of each signal and the accumulated signal. The tabulated data show that the fractions become more aromatic as the number of carbon atoms increases. The heavier cuts are the main sources of condensed aromatic species thus triaromatics are then observed after C18. Tricyloparaffins are higher for C14 and tend to reduce as the carbon number increases, result that is not logical and confirms the above mentioned limitations.
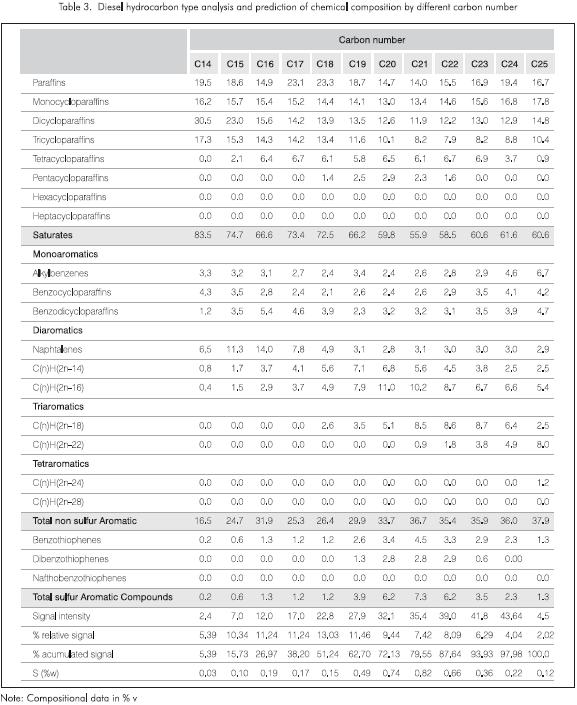
In spite of these limitations, the information in this table is very interesting since it shows the concentration of species per carbon number and the incidence of the cut in the diesel as a whole. It is then possible to define the fractions that must be treated to generate a diesel with the desired specifications.
Table 4 shows the results of the analysis of the accumulated signal from the beginning (end of C13) up to each one of the selected carbons and the distribution of the components in terms of the final boiling temperature of each fraction (normal boiling temperature of the largest n-paraffin in the selected range). The first column corresponds to the integration of the signal from the end of C13 up to the end of C14, the second one constitutes the average of the signal between the end of C13 up to the end of C15 and so forth. The calculation of the chemical composition along the boiling range assumed in this case, the same response factor for all the components over the entire range of carbon numbers as well as no overlaps between compounds of different carbon number.
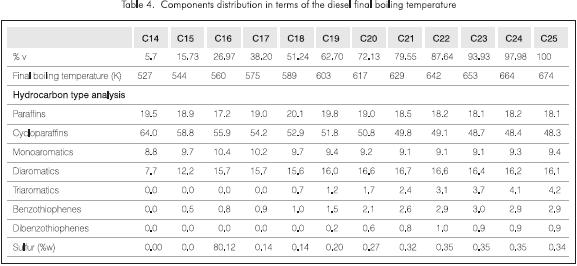
The final balance of the data from the last column in Table 4 approximately matches the results given in Table 2. Nevertheless, there are important differences for the triaromatics and the sulfur species. These deviations may be due to problems in assigning exact masses, limitations of the assumption of equal response factors for all the peaks, overlaps between different carbons in the gc run and errors in the quantification of the signal corresponding to each carbon number. This issue will be covered in a later research. Another reason for the discrepancy is that the HC22 method does not take into account all possible species that become astronomical as the carbon number increase, therefore these errors would be larger for higher carbon numbers.
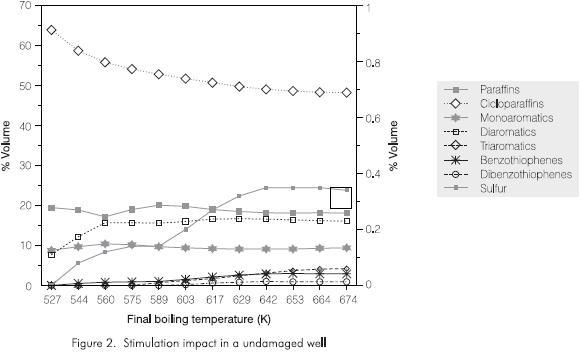
The graphic representation of these results is shown in Figure 2 which gives the volume percentage of each component in the cut in terms of their final boiling temperature. This graph clearly shows that sulfur contaminants and the triaromatic species mainly concentrate in the fractions distilling above 589 K (equivalent to 50% volumetric yield). On the other hand, a decreasing trend can be observed in cycloparaffins as the final temperature of the cut increases. The observed tendencies in aromatics and sulfur compounds but not in cycloparaffins are consistent with the results obtained from the analysis of the cuts (Nero et al., 1991).
CONCLUSIONS
-
The proposed method allows a thorough analysis of the chemical composition of the diesel and of the distribution by hydrocarbon type (saturated hydro- carbons, aromatics and sulfur compounds) in terms of their final boiling temperature without the need to obtain distillation cuts of the problem sample.
-
The new proposed methodology, which will be validated through a complete research of the cuts, offers important advantages over the traditional procedure because it could be used to separate the whole spectra of the diesel in terms of different carbon number allowing the construction of curves of components distribution througout the boiling range of the sample, from data obtained in a single analysis. When the sole interest is to obtain the chemical composition of the sample, the main disadvantages of the new procedure are that it is less accurate and more time consuming than the traditional one. In principle, both disadvantages could be reduced by changing the chromatographic conditions to speed the analysis time and by reducing the sweep rate of the magnet to obtain more accurate results.
-
The observed data clearly demonstrate that the results obtained through the new technology are similar to the ones obtained through the traditional sample injection method; nevertheless, some data corrections must be introduced to take into account effects of differences in chromatographic response factors and overlaps of compounds of different carbon number.
-
The observed trend in condensed cycloparaffins concentration indicates that it would be necessary to change mass spectra adquisition conditions to improve mass assignment in order to get more reliable results with the HC22 method.
REFERENCES
Fisher, I. P. and Fischer, P., 1974. "Analysis of high boiling petroleum streams by high resolution mass spectrometry", Talanta, 21: 867 - 875. [ Links ]
Gallegos, E. G., Gree, J. W., Lindeman, L. P., LeTourneau, R. L. and Teeter, R. M., 1967. "Petroleum Group Type Analysis by high resolution mass spectrometry", Analytical Chemistry, 39: 1833. [ Links ]
Nero, V. P., MacMahon, C. S., Popiel, E.J., Chapman, G. L. and Chao, S., 1991. "A comprehensive analysis of diesel fractions", Symposium on Modern Analytical Techniques for the Analysis of Petroleum, presented before the Division of Petroleum Chemistry, Inc. American Chemical Society, New York City Meeting. [ Links ]
Robinson, C. J., 1971. "Low resultion mass spectrometric determination of aromatics and saturates in petroleum fractions", Analytical Chemistry, 43 (11): 1.425 - 1.434 [ Links ]
Sink, C. W., Hardy, D. R. and Huang, M. A., 1994. "An accurate hydrocarbon type analysis of all fuel types", Fuel Science and Technology Int. 12 (7 y 8): 1.081 - 1.103. [ Links ]
Ullman, T. L., Mason, R. L. and Montalvo, D.A. "Effects of fuel aromatics, cetane number, and cetane improver on emissions from a 1991 prototype heavy-duty diesel engine", SAE Technical Paper Series 902171. [ Links ]
Wilson, M. F., Fisher, I.P. and Kriz, J. K., 1986. "Cetane improvement of middle distillates from Athabasca Syncrudes by Catalytic hydroprocessing", Ind. Eng. Chem. Prod. Res. Dev., 25: 505 - 511. [ Links ]














