Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383On-line version ISSN 2382-4581
C.T.F Cienc. Tecnol. Futuro vol.1 no.4 Bucaramanga Jan./Dec. 1998
BIOTRANSFORMATION AND REMOVAL OF SULFUR FROM DIBENZOTHIOPHENE USING IMPROVED BIOCATALYTIC METHODS
ABSTRACTT
Three methods for the removal of sulfurfrom dibenzothiophene were evaluated using biocatalytic processes. The methods were a microbial, an enzymatic and a combined one that involves a previous enzymatic oxidation followed by microbial degradation. The byconversion was evaluated over the molecular dibenzothiophene model, obtaining higher byconversion percentages through the combined method. The microorganisms used in this study correspond to several Colombian indigenous strains isolated by direct methods from natural sources, and using a standard strain as positive control. All strains have shown sulfur removal capacity, and organic solvent tolerance. The enzyme used was a hemoprotein with peroxidase activity, Cytochrome C, obtained from equine heart.
Keywords: dibenzothiophene, sulfur-removal, hemoproteins, peroxidase, cytochrome C, biodesulfurization.
RESUMEN
Tres métodos de remoción de azufre a partir de dibenzotiofeno fueron evaluados empleando procesos biocatalíticos tales como byconversion microbiológica, enzimática y un método combinado que reúne la preoxidación via enzimática y la posterior degradación microbiana. La biodegradación fue evaluada sobre el modelo molecular dibenzotiofeno, encontrándose un mayor porcentaje de byconversion empleando el método combinado con respecto a los otros dos métodos. Los microorganismos evaluados corresponden a varias cepas colombianas aisladas por métodos directos de fuentes naturales y una cepa patrón como control positivo. Todas las cepas han presentado capacidad biodesulfurizadora y tolerancia a medios orgánicos. La enzima empleada corresponde a una hemoproteina con actividad peroxidasa, Citocromo C de corazón equino.
Palabras clave: dibenzotiofeno, extracción-azufre , hemoproteínas, peroxidasa, citocromo C, biodesulfurización.
INTRODUCTION
Sulfur compounds present in fossil fuels and crude oils, represent an important problem in the charcoal and petroleum industries. Without a proper treatment carried out during the refining and production processes of petroleum products the fossil fuels can generate corrosion and pollution problems. Sulfur compounds contented in crude oil can vary considerably, and their properties and features are reflected on the source, aging, and maturity of the crude. Common methods employed for inorganic sulfur removal have chemical and physical features that make them inappropriate for their application on organic sulfur removal. The presence of sulfur in petroleum can cause the loss of its quality and in addition, serious environmental problems like acid rain.
The use of biotechnological tools is a new step in advanced methods for petroleum desulfurization processes (Monticello, 1993; Kilbane and Woodstock III, 1991). Some research groups have found microorganisms with a high desulfurization capability (Ohshiro et al, 1995; Krawiec, 1990). These microorganisms can do the C-S bond cleavage, releasing sulfur and allowing the complete mineralization of organic sulfur transforming it into sulfate by aerobic mechanisms or into dihydrogen sulfide by anaerobic mechanisms (Yamada et al, 1968; Kayser Qt al, 1993; Monticello, 1993; Monticello, 1994; Ózbas etal, 1996).
Nevertheless, some of the metabolic pathways that the microorganisms use involve half-reactions and production of undesirable compounds that are characterized by ring cleavage and no sulfur release (Oldfield et al, 1997; Kim et al, 1990; Olson et al, 1993). A complete or a high percentage of organic sulfur removal can be achieved by using biotechnological tools such as biocatalysis and microbial desulfurization processes. These processes were first applied on thiophenic sulfurs because they are the most common and representative molecular models of the sulfur-heterocyclic compounds in petroleum. Some hemoproteins, as well as microbial cells, are known for their ability to biocatalyze the oxidation and mineralization of sulfur compounds such as dibenzothiophene (DBT) present in the heavy fractions of petroleum. The use of enzymes and specially of hemoproteins with peroxidase activity such as Cytocrome C in previous works (Vazquez-Duhalt et al, 1993; Fedorak et al, 1993; Hartdegen et al, 1984.), has shown a high sulfur conversion activity. It is possible that a combined process that involves an initial biotransformation of dibenzothiophene by these enzymes could increase the rate of the biodesulfurization process itself, due to the fact that the microorganisms can metabolize this products in a more efficient way than the usual one. As a result, the metabolites then become more bioavailable compounds for the microorganisms, which can finally cause the mineralization of the organic sulfur.
MATERIALS AND METHODS
Chemicals
Dibenzothiophene was obtained from Merck Co. (USA), 2-hydroxibiphenyl, dibenzothiophene sulfone and biphenyl were obtained from Aldrich Co. (USA). The oxidizing reagent, hydrogen peroxide was obtained from J.T. Baker Co. (USA). Equine heart Cytochrome C (A grade) was obtained from Sigma Co. (USA).
The microorganisms
The microorganisms used in this work were Colombian indigenous strains isolated from natural sources (water and soil) by direct methods and a standard strain with a proven capacity for desulfurization Rhodo-coccus rhodochrous IGTS8 (Sorkhoh et al, 1990; Kilbane, 1992), was obtained from the ATCC collection No.53968. All strains were cultivated in a medium containing: glycerol 5% (w/v), glucose 5·103 g·m-3,Na2HP04 1 g·m-3, KH2P04 0,5·103 g·m-3, NH4C1 1·103 g·m-3, NaN03 1·103 g·m-3, MgCl2·4H20 1,3% (w/v), FeCl3·6H20 1,3% (w/v) and CaCl2·6H20 3% (w/v). This media corresponds to a modification of the original media suggested by Kilbane and Bielaga (1990).
The screening of the microorganisms
The microorganisms were evaluated by means of a biodesulfurization assay. The applied evaluation criteria was the qualitative and quantitative detection of metabolites obtained from the biodesulfurization metabolic pathway. The qualitative evaluation was conducted by determining the presence of a fluorescent metabolite, such as 2-hydroxibiphenyl in the culture plates. Two methodologies were compared for the preliminary evaluation of the strains: (A) substrate exposition to the dibenzothiophene from the time of the inoculation, and (B) cell pregrowth under limited sulfur conditions, and final exposure to dibenzothiophene. All the strains were precultivated during 72 hours using the above described media, afterwards, a sample of each strain was taken and inoculated into 9·106 m3 of fresh media and 1·106m3 of the sulfur source, for a final concentration of the organic sulfur compound of 10 g·m3 or 100 g·m3. The culture was incubated during 15 days, in a controlled temperature room at 303 K. Finally, the cultures were acidified with Hydrochloric acid and the metabolites were extracted for analysis.
Biocatalytic desulfurization
The enzymatic trials were carried out with equine heart Cytochrome C (A grade) obtained from Sigma Co. in a 5O·106m3 reaction tube with magnetic stirring and using 10 g·m-3 and 100 g·m-3 as final concentrations of Dibenzothiophene, and a concentration of 33,81 nM of the Cytochrome C equine heart. The reaction media used remained constant, a solution 10% (v/v) ethanol in water (binary system), where the water phase is represented by 60 mM Citric acid - disodium monobasic phosphate buffer pH 6.1. The reaction was carried out at 293 K - 295 K and was started by adding hydrogen peroxide (IX). This addition was continued until the final concentration of (5X) 0,5 nM was attained. After 1 h, the reaction was finally stopped with the addition of 0.1·106m3 of a 6 N hydrochoric acid solution. The enzymatic reaction was also carried out with negative controls constituted for reaction mixtures: A (enzyme and no-peroxide), B (peroxide with noenzyme) and a reaction blank with neither enzyme nor peroxide. All the assays were made separately, using the enzymatic process only when the biocatalytic degradation was proven.The combined method was developed afterwards
Analytical methods
The quantitative determination of the metabolites was carried out by High Pressure Liquid Chromatography (HPLC). The chemical analysis of the samples was made employing destructive assays in which the products of the microbial or enzymatic metabolisms were separated from the water phase by liquid-liquid extraction with dichloromethane as the solvent. Each 10·106m3 sample was treated with 5·106m3 of n-hexane (in triplicate), The fractions were pooled and then concentrated to a final volume of 1 ·106m-3 in a nitrogen atmosphere. The identification and quantification of the metabolites present in 10·106m3 of organic extract, was made via HPLC analysis using a Hewlett Packard 1090 equipped with an UV detector. The chromatographic analysis of the metabolites was conducted using a silica fused capillary column with a reverse phase of polimethylsiloxane (HF-5-C18, Perkin Elmer)), 0.25 m* 4.6·103m I.D. * 0.5·10-6 m. The solvent was a mixture of water-acetonitrile, 70:30, with acetonitrile gradient up to 100% acetonitrile and a flow rate of 1·106m3min-1. The subproduct quantification was made using the external standard method.
RESULTS AND DISCUSSION
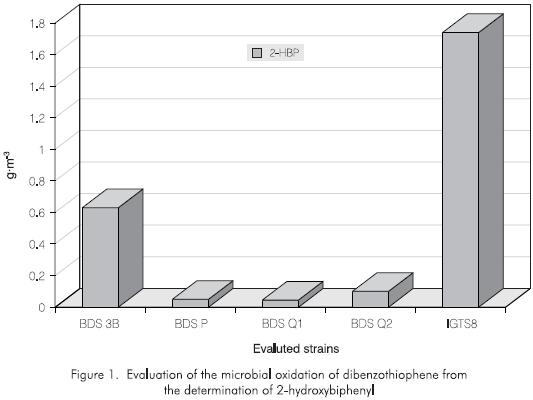
Of a total of eleven strains evaluated using plate cultures, only four strains were preselected, based on the fluorescent presence of 2-hydroxybiphenyl (2-HBP) revealed by UV light. The chemical analysis of the liquid cultures confirmed the presence of such compound indicating that organic sulfur metabolism was reached in the absence of an inorganic sulfur source. This inductive effect has been mentioned by other authors such as Gallagher et al, (1993). The analytical methodology could allow the quantification of sulfur traces obtained from the inorganic salts used in the culture media and the sulfur present in the inoculum itself before the cells were exposed to the DBT In one case, when the DBT was added to the medium, the cells were found in a starving sulfur state because the element had been consumed, the DBT then became the main sulfur source. In other cases, the DBT was added employing additional sulfur traces of the medium which produces an inductive effect on the DBT metabolism. In the first case, it was observed that four strains were selected in the cultures in which the 2-HBP was detected. The positive control strain, Rhodococcus rhodochrous IGTS8, showed that the metabolite 2-HBP was produced in both cases, but only concentration was only higher in the latter one. No strains showed the production of Dibenzothiophene sulfone (Figure 1).
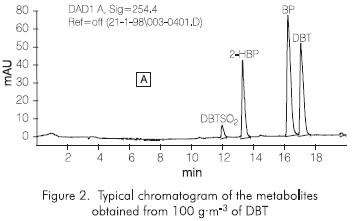
In the enzymatic oxidative effect over the DBT substrate using Cytochrome C (Vazquez-Duhalt et al, 1993), the production of three main products has been reported: dibenzothiopene sulfone (DBTS02), 2-hydroxybiophenyl (2-HBP) and biphenyl (BP). We also observed (unpublished work) a percentage of unknown metabolites that we presume correspond to oxided forms of DBT (Figure2). The chromatographic results obtained for each one of the expected subproducts in the enzymatic reactions with Cytocrome C, over two different concentrations ofDBT(10 g·m-3) and 100 g·m-3, have shown a similar production of the oxided compound (DBTS02), and the non-oxided compound (BF) in both cases, but the production of HBP was not observed (Figures 3 and 4). These results clearly show that the enzymatic process has a positive effect for the conversion of the sulfur substrate employed. This positive effect is based on the bioavailability of a substrate or a sulfur source in an organic media.
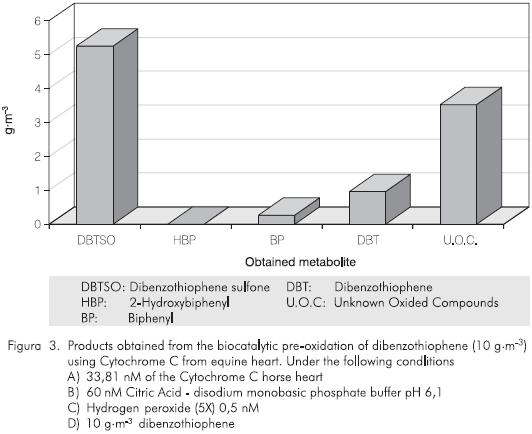
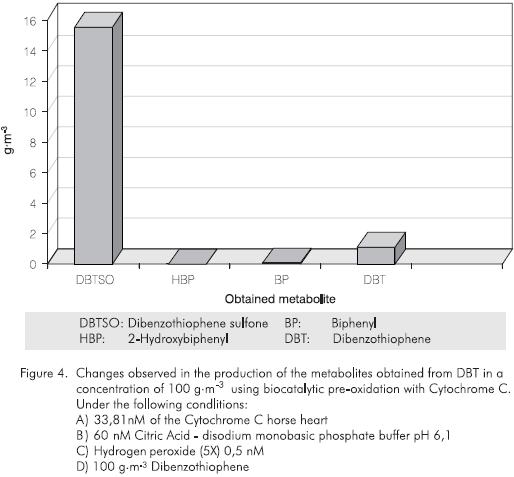
The treatment with Cytochrome C or by means of any other oxidant agent could produce less organic soluble metabolites that allows a higher transport rate (high partition coefficient) into the cells. In this case, the metabolites will migrate to the aqueous phase which make them much more available for the microorganisms to continue the biodesulfurization process. This confirms that the enzymatic oxidation can produce a high percentage of conversion of sulfur into inorganic compounds without decreasing the caloric power of crude oils due to the efficiency and specificity of the enzymatic process.
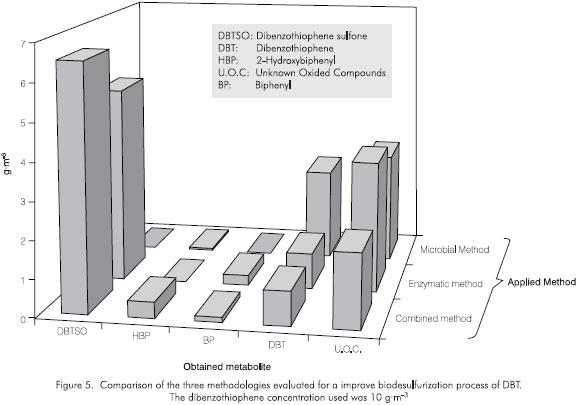
The preliminary results for the application of a combined method (enzymes plus microorganisms) have shown a reduction of the DBT concentration of up to 60%, with an enhancement of the oxided and non oxided metabolites concentration of almost 80% (evaluated over the final concentration of sulfone), for an initial DBT amount of 100 g·m-3. In this case the enzyme and peroxide amounts were adjusted to prevent a possible inhibitory effect caused by the substrate concentration. It should be noted that the strain BDS 3 which showed the highest HBP production was not the same strain that presented the highest sulfur removal efficiency in the combined method, BDS P (Figures 1 and 5). The production yields are shown in Figure 5, to allow the comparison of the evaluated methods using the strain BDS P with a DBT concentration of 10 g·m-3.
CONCLUSIONS
-
The ability of some mesophylic bacteria for the desulfurization of dibenzothiophene was evaluated by two methodologies in which the metabolite 2-hydroxy-biphenyl was determined both by optical fluorescence and with chemical analysis.
-
The highest percentage of DBT biotransformation was reached in the combined biocatalitic process involving preoxidation with the hemoprotein Cyto-chrome, and a post-microbial treatment (biodegradation).
-
These results may be improved with the employment of chemically modified enzymes which could potentially enhace the BDS process working in organic solvent systems and non-water systems, as well as with other hemoproteins with peroxidase activity such as Chloroperoxidase.
ACKNOWLEDGMENT
The authors wish to thank the biorefining research group from the Instituto Colombiano del Petróleo for its valuable assistance and help during the course of this work.
REFERENCE
Crawford D. L. and Gupta R. K., 1990. "Oxidation of dibenzothiphene by Cunninghamella elegans". Curr Microbiol, 21: 229-231. [ Links ]
Fedorak, P. M, Semple K. M, Vázquez-Duhalt, R. and Westlake D. W., 1993. Enzyme Microb. Technol., 15 (May): 429 - 37 [ Links ]
Gallagher, J. R, Olson, E. S. and Stanley, D. C, 1993. Microbial desulfiirization of dibenzotiofene: a sulfur-specific pathway, FEMSMicrobiology Letters, 107: 31-36. [ Links ]
Hartdegen, E. J., Coburn, J. M. and Roberts, R. L, 1984. "Microbial Desulfurization of Petroleum", CEP. [ Links ]
Izumi, Y, Ohshiro, T., Ogino, H., Hine, Y. and Shimao, M., 1994. "Selective desulfurization of dibenzothiophene by Rhodococcus erythropolis D-1", Appl. Microbiol Biotechnol., 60: 223 -226. [ Links ]
Kayser, K. J., Bielaga, B. A., Jackowski, K., Odusan, O. and Kilbane II, J.J.1993. "Utilization of organosulfur compounds by axenic and mixed cultures of Rhodococcus rhodochorous IGTS 8", J. Gen Microbiol, 139: 3123-3129. [ Links ]
Kilbane, J. J. and Bielaga, B.A. 1990. "Toward sulfur-free fuels", Chemtech, 20: 747-51. [ Links ]
Kilbane II J. J. and Woodstock III, 1991. "Mutant Microorganisms useful for cleavage of organic C -S bonds", Patent 5104801. [ Links ]
Kilbane II, J. J and Jackowski, K. 1992. "Biodesulfurization of water-soluble coal -derived material by Rhodococcus rhodochorous IGTS", Biotechnol Bioeng, 40: 1107 -1114. [ Links ]
Kim, H. Y, Kim, T S. and Kim, B. H., 1990. "Degradation of organic sulfur compounds and the reduction of diben-zotiophene to biphenyl and hydrogen sulfide by Desul-fovibrio desulfuricans Mb", Biotechnol lett. 12: 761 -764. [ Links ]
Krawiec, S. 1990. "Bacterial desulfurization of thiphones: screening techniques and some speculation regarding the biochemical and genetic", Dev. Ind. Microbiol., 32: 103-114. [ Links ]
Monticello, D. J., 1993. "Biocatalytic desulfurization of petroleum and middle distillates", Environmental Progress, 12 (1): 1-4. [ Links ]
Monticello, D. J., 1994. "Biocatalytic desulfurization", Hydricarbons Processing (February). [ Links ]
Ohshiro, T, Hirata T. and Izumi, Y, 1995. "Microbial desulfurization of dibenzothiophene in the presence of hydrocarbon", Appl. Microbiol Biotechnol., 44: 249 -252. [ Links ]
Olson, E. S., Stanley, D. C. and Gallagher, J. R, 1993. "Characterization of intermediates in the microbial desulfurization of dibenzothiophene", Energy & Fuels, 7: 159-164. [ Links ]
Oldfield, C, Pagrebinsky, O.,Simons, J., Olson, E. S. and Kulpa, CH. F 1997. "Elucidation of the metabolic pathway for dibenzothiophene desulfurization by Rhodococcus sp. strainIGTS8(ATCC53968)", Microbiology, 143: 2961-2973. [ Links ]
Ózbas, T., Durusoy, T, Erincin, E. and Yürüm, Y 1996. "Optimization of the growthparameters of Rhodococcus rhodochorous, a sulfur - removing", Fuel 75 (13): 1556 -1600. [ Links ]
Sorkhoh, N. A., Ghannoum, M. A., Ibrahim. A. S., Stretton, R J. andRadwan, S. S.,1990. "Crude oil and hydrocarbon-degrading strain of Rhodococcus rhodochrous isolated from soil and marine environments in Kuwait", Environment Pollution, 65: 1-17. [ Links ]
Vázquez-Duhalt, R., Westlake, D. W S. and Fedorak P. M., 1993. "Cytochrome C as a bicatalyst for the thiophenes and organosulfides", Enzyme Microb. Technol., 15 (June): 494-499. [ Links ]
Yamada, K, Minoda,Y, Kodama, K, Nakatani, S. and Akasa M, T. 1968. "Microbial conversion of petro-sulfur compounds. Part I. Isolation and identyification of Dibenzothiophene utilizing Bacteria", Agr. Biol . Chem., 32 (7): 840 - 845. [ Links ]














