Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383On-line version ISSN 2382-4581
C.T.F Cienc. Tecnol. Futuro vol.2 no.2 Bucaramanga Jan./Dec. 2001
ABSTRACT
In this study, ozone efficiency on the treatment of wastewater contaminated with high amounts of spent caustic (byproduct from hydrocarbons sweetening processes), is evaluated. The type of feedstock, pH, gas/liquid ratio and influence of catalyst use, are analyzed, in addition to the contact system used for the experiments. As a main conclusion, the feasibility of ozone in the treatment of industrial residues was established, especially in wastewater with high phenol contents.
Keywords: ozone, spent caustic, phenol, chemical oxidation of pollutants.
RESUMEN
Este estudio evalúa la eficiencia del ozono en el tratamiento de aguas residuales contaminadas con altas cantidades de soda gastada (subproducto de los procesos de endulzamiento de hidrocarburos). Se analiza el tipo de carga, el pH, la relación gas/líquido y el uso de un catalizador, además del sistema de contacto utilizado en los experimentos. Como conclusión principal, se estableció la viabilidad del tratamiento con ozono, de residuos industriales, especialmente en aguas residuales con altas cargas fenólicas.
Palabras clave: ozono, soda gastada, fenol, oxidación química de los contaminantes.
INTRODUCTION
A refining complex is made up of a variety of production processes including distillation, catalytic cracking, visbreaking, petrochemistry, and sulfur recovery, among others. As a result of these processes, waste-water is generated, containing a great variety of pollutants (hydrocarbons, phenols, mercaptans, sulfurs, ammonia and others), which can remain in the water in free, emulsified or dissolved forms.
The spent caustics generated in the hydrocarbons sweetening processes and the sour waters from the steam stripping systems are discharged into wastewater treatment systems, characterized by their high phenol compounds content and for having a high concentration of organic matter, sulfur and cyanide. This wastewater must be treated in order to reach the appropriate quality to be discharged with minimum impact on the environment or to be reused.
Although the pollutants that are not dissolved can be removed by physical separation processes, sedimentation or flotation (Forero et al, 1999), the remain of the dissolved or emulsified compounds such as residual hydrocarbons, phenols and gases, among others, can be removed almost exclusively by biological or chemical processes.
Biological processes for the treatment of this type of effluents have been reported for many years and it has been proved that for the biomass to eliminate refractory compounds, a long reaction period is required (Rebhun et al, 1994). In the case of phenol, efficient removal is reported only after prolonged residence time and phenol concentrations under 200 mg/L. Also, the biological processes applied on typical refinery waste-water have several disadvantages that notably reduce removal rates, such as the inhibitory effects of complex phenol compounds, the marked effect on stability and efficiency produced by high concentrations of solids and hydrocarbons and uncontrolled discharges of concentrated phenol streams from treatment plants, which interfere with the biological process up to the point where they are cut off completely (Rebhun et al, 1994).
Some solutions that can be adopted to protect and maintain the biological process for refinery wastewater are: the flow control of unexpected streams like those of unsegregated rainwater and those that come from contingency work at process plants, the efficient removal of suspended solids and hydrocarbons before biological treatment and the segregated treatment of low flow streams that have a high concentration of pollutants, as is the case of spent caustics.
This work was carried out for this last option, seeking to evaluate the technology of oxidation based on ozone, to treat low flow streams and streams with high phenol concentrations separately, which may represent up to 50% of the total phenol contamination levels in refinery wastewater streams (Forero et al, 2000).
Conventionally, streams with high phenol content are diluted in industrial waste waters before being send to adaptation processes that include primary sedimentation, neutralization and stripping, flotation and stabilization, and air oxidation in order to obtain an effluent that is adequate for biological treatment. Nevertheless, although the above processes tend to improve the biodegradability of these waters, they limit the possibility of reuse, due to the high salinity of the effluents (Berne, F, 1995).
Due to these inconveniences and limitations, ozone treatment is proposed as an effective alternative in the elimination of phenol compounds in segregated streams (spent caustic), which would mean additional advantages over the general treatment of industrial waste-water, such as:
- Improvement in biological process stability due to the fact that overloads in the system are eliminated and toxicity is decreased with ozone pretreatment.
- Decrease in biological treatment costs.
- Opportunity to reuse high volumes of treated wastewater streams, with low contents of dissolved solids.
- Decrease in the volume of spilled wastewater, along with an increase in final effluent quality.
- Makes the operation more flexible and prevents modifications to increase the size of biological treatment systems.
GENERAL INFORMATION
Ozone is an allotropic (unstable) formula of oxygen in which three atoms are combined to form a new molecule. Due to its instability, at alkaline values of pH it quickly decomposes generating highly reactive free radicals. The ozone's oxidation potential (-2.7V) is greater than that of the hypochlorite ion (-1.49V) or chlorine (-1.36V), substances widely used in wastewater treatment such as oxidants. Ozone is surpassed only by the hydroxyl radical (· OH) and fluoride in its oxidation capacity.
This indicates that ozone has more effective action than chlorine in water treatment. Furthermore, chlorine has a great tendency to form trihalomethanes (THMs), which have negative consequences on the environment. On the other hand, ozone has an exceptional advantage over any other oxidant and that is the fact that whatever is treated with ozone is not loaded with unwanted chemical byproducts. If the organic pollutant is completely mineralized, residual products would be C02 and H20, and others that comes from compounds such as H2S04, HC1, HN03 and others.
Ozone also has advantages in applications for combined wastewater treatment processes. Previous oxidation with ozone favors biological post-treatment because substrate toxicity is decreased, as has been evaluated in the case of phenol and its products from oxidation with ozone (Trapido et al, 1999).
Recent literature constantly mentions that one of the disadvantages of using ozone is the high cost. Nevertheless, during the last few years, technological development of equipment to produce ozone has been remarkable, making it a competitive system of residue treatment, especially in conditions of severe contamination (Blaich, L.,1999).
OZONE REACTIVITY
Ozone in an aqueous solution reacts with most pollutants in industrial wastewater in two different ways (Figure 1):
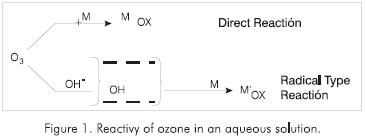
-
By direct reaction of the molecular ozone.
-
By reaction of the species formed by ozone decomposition in the water (free radicals), (Masten, 1994; Hoigné and Bader, 1977).
In a reaction that follows the radical path, the mechanism is based mainly on the formation of hydroxyl radicals. There are three kinds of substances that can affect the process of free radical reaction, which are: initiators, promoters and inhibitors.
Initiators: are compounds capable of inducing the formation of a super oxide anion (02-) from an ozone molecule. These compounds can be inorganic (hydroxyl ions, hydroperoxide ions and some cations) or organic (glyoxalic acid, formic acid or humic substances). Ultraviolet radiation is another form of initiation of the mechanism by radicals along with the H202/03 combination. The last two systems form part of the "advanced oxidation" processes.
Promoters: of free radical reactions are all of the organic molecules capable of regenerating the super oxide anion (02-) from a hydroxyl radical. The most common organic promoters are the compounds that include aromatic groups, or double bonds. The phosphate species are among the main inorganic promoters.
Inhibitors: are compounds that are capable of consuming -OH radicals without regenerating the super oxide anion (02-). The most common inhibitors are carbonate and bicarbonate anions, the alkyl groups, tertiary alcohols and humic substances.
A free radical process is less selective than a direct reaction process. Nevertheless, it is faster, which is definitely more important in a wastewater treatment process. Therefore, the elimination of inhibitors is a very important factor to be considered during the course of the reaction, in order to reduce ozone consumption.
The oxidation of the chemical species in the water on behalf of the ozone is of special interest in the work carried out. Ozone activity is especially convenient for the treatment of chemical species that contain nu-cleophilic sites characterized by the presence of O, N, S or phosphorous. Thanks to ozone activity, substances such as amines, pesticides and aromatic species can be transformed and taken to a state in which their biodegradability is facilitated.
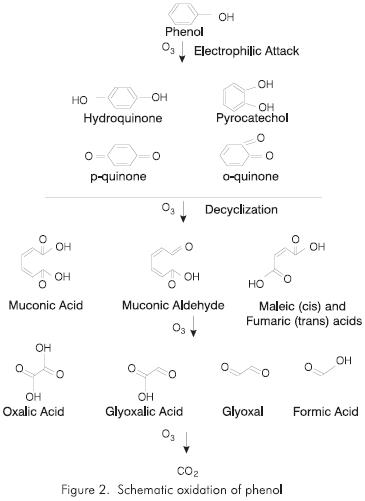
Phenols are a group of potential substances among compounds that are susceptible to ozonation. Phenol products (phenol, compounds substituted by phenol, quiñones and polyphenols) are characterized by the presence of an OH donor group above the aromatic nucleus. This group makes them strongly reactive with ozone (Masten, 1994).
The direct reaction between phenol and ozone follows a reaction of the second order, although this assumption is not always exact (Gurol, 1982).
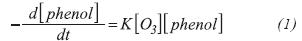
The products generated in this reaction depend on its intensity. If the aromatic ring is oxidized, diphenols and quiñones result. When the ring is broken by the ozone activity, muconoic acids and their derivatives are generated, and the ozonolysis of these substances produce oxalic acids, glyoxalic acid and formic acid as well as glyoxal. These reactions are summarized in the diagram shown in Figure 2.
Whatever the mechanism in the reaction, the oxidation products are the same. Nevertheless, the efficiency of the reaction can be improved by advanced oxidation processes including ultraviolet radiation (UV). In this case, the following order of efficiency in reactivity is proposed (Gurol, 1987).
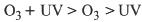
Depending on their structure, the reaction of phenol compounds increases when the electronic density in the aromatic ring is greater, and decreases with the size of the substitute (Gurol, 1987). It has also been shown that the reaction rate increases at a high pH value due to ozone autodecomposition and subsequent generation of free radicals (Hydroxyl radicals), these free radicals have high reactivity (Staehelin and Hoigne, 1982). Reaction rate is also increased at high pH values by the phenol dissociation in phenolates ions, such ions have higher reactivity than phenol. Although the reaction rates measured in the work carried out present certain variations, values of the following order can be considered (Hoigne and Bader, 1983):
- 1.3 *103 M-1 S-1 for non-dissociated phenol
- 1.4 HO9 M-1 S-1 for the phenate ion
Other fundamental parameters to be considered are:
pH: It has been proven that a decrease in pH increases ozone stability and therefore, the ozone dose to be applied must be increased, due to the low reaction rate.
Required Dosage: The ozone dose required to oxidize phenol is generally determined by experiment, although in most cases, a few mg 03/L of wastewater are required.
Residence Time: Optimum contact time depends basically on the objective of ozonation. In general, the reactions of ozone oxidation with phenols occur in a short time. In the pilot scale assays, they take a maximum of six minutes to go through the system.
Feeding Gas: Pure oxygen or atmospheric air can be used, but over 99% of the systems that use ozone for water treatment use air as the feeding gas. The air fed may contain impurities: humidity and particulate matter, for this reason it should be prepared before entering the system.
METHOD
Wastewater used in the experiments
The aqueous phase was obtained from spent cresylic caustic, previously treated by means of a controlled neutralization - extraction process (separation and removal of cresylic acids). To reduce concentration to 2000 mg/L of phenol (Forero et al, 2000). The pH of the solution was kept constant throughout the test at an interval of 9-10 with addition of concentrated caustic solution.
Ozone treatment
The ozonation were carried out in the system shown in Figure 3. A given volume of a sample is introduced in the tank, where it is taken and recirculated through a tubular reactor made of transparent PVC 2.54 cm (1") in diameter and six m long, by means of a pump at a flow rate of 30 L/min and apressure of 1.448 Bar (21 psig).
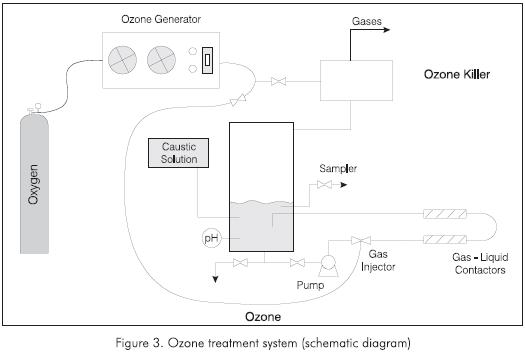
Ozone was produced with an OZONAIR generator, which is fed with pure oxygen from a cylinder that ensures a constant supply throughout the experiment. A production of 19 grams of ozone per hour is reached in a proportion of approximately 4% volume of the total ozone-oxygen mixture. This is the typical mixture used for all of the experiments.
The ozone was put into contact with the aqueous phase by means of a system designed at the Instituto Colombiano del Petróleo (ICP) that reaches high degrees of dispersion, gas bubbles with small diameters and low energy consumption, to ensure high mass transfer (Forero et al, 1999).
The experiments were carried out until a concentration of 10% of the initial value of the substrate was reached. This value corresponds to an approximate phenol concentration of 200 mg/L, from which a biological treatment process can begin.
Analytical methods
Ozone generation and losses in the storage tank were determined by iodometric method 2350E of Standard Methods.
Throughout the experiment, 20 mL samples were taken at defined intervals from the center of the storage tank that was always completely agitated and homogeneous. Phenol content and chemical oxygen demand (COD) of the samples were determined in agreement with procedures 5530D and 5220 of the Standard Methods respectively.
Parameters Evaluated
The parameters that were varied in this study were:
- - Type of feed: industrial wastewater (IWW) and synthetic wastewater (SWW).
IWW: industrial wastewater sample.
SWW: synthetic wastewater sample.
- The effect of the catalyst (metallic oxides) on the system to increase ozone reactivity.
- Gas/Liquid ratio in the tank.
- pH.
The synthetic phenol sample was prepared dissolving analytical phenol (99% purity) in distillated water to obtain the desired concentration.
RESULT AND ANALYSIS
Figure 4 shows the effect produced by the variation in type of feed in the ozonation system, by submitting it to ozone stream of 19 g/h. The decrease in phenol is represented in the curve SWW in relation to time in the synthetic sample solution. The curve IWW, represents the same treatment, but carried out on industrial waste-water sample, and the curve IWW+Catal represents, the behavior of the industrial wastewater sample in the presence of a catalyst (metallic oxide).
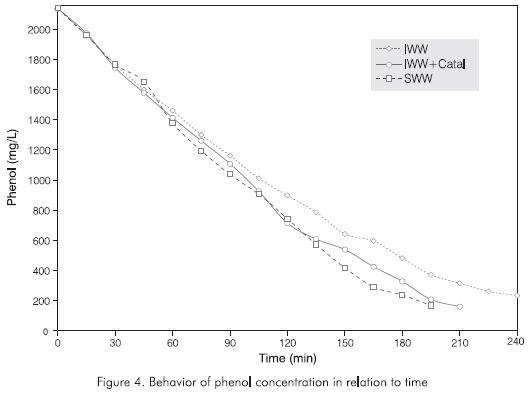
The diagram shows that during the first 60 minutes, the three samples have the same behavior of phenol decreasing by approximately 35%. There is a deviation in the behavior of phenol degradation for industrial waste water samples: IWW and IWW+Catal, due to the fact that the SWW matrix is free from inhibitors or its effect on the system is minimal, the deviation suggests the presence of substances interfering in IWW sample that alter the phenol reaction with the ozone, or begin to interact with it before the phenol compounds are degraded.
To reach the desired phenol value (200 mg/L), the synthetic sample requires approximately 180 minutes of ozone treatment. If you compare the time needed to reach a phenol concentration of 200 mg/L in the three experiments, it is observed that the ozone has a greater impact on the degradation of synthetic waste water sample (SWW) than on the solution of spent caustic (IWW and IWW+Catal). In the experiment for IWW, over 240 minutes are required to reach a final 200 mg/L phenol, which represents an additional ozone consumption of over 25%. Nevertheless, the application of the catalyst on the tests counteracts against the effect of the inhibitors and the desired phenol concentration is reached in a times similar to the synthetic samples (SWW). The differences in phenol degradation times are due mainly to three factors:
- The presence of organic substances in addition to phenol, which contribute greater ozone demand to the reaction system.
- The action of radical inhibitors, such as bicarbonate and carbonate ions, which are common in this type of waters (Langlais, 1991).
- The increase in structure complexity of phenol compounds, which decreases reaction rate regarding ozone (Berné, 1995).
Table 1 shows the values of the consumed g 03/ degraded g phenol (ø) ratios, for different time intervals, varying phenol ratio and pH. It can be seen that the highest ø ratios reach a value of 1.92, and the lowest reach 0.78. This gives us an idea of the efficiency of the mass transfer system, because it quantifies the effectiveness of the ozone injected into the reactor and prevents its loss in the tank. For the case explained, the efficiency of the transfer system is quite high in relation to the values reported in previous works, where ø's of approximately 2.5 g 03/g of removed phenol are reached (Gurol, 1987).
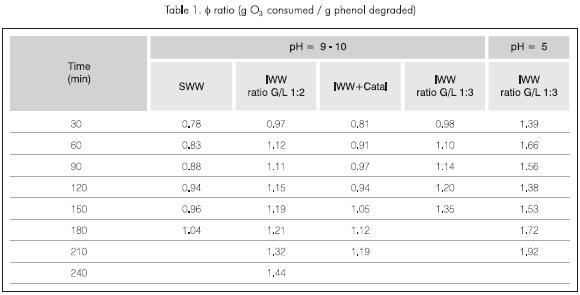
It is also observed that there is a more efficient use of ozone for the SWW case. That is, less ozone is consumed for the same phenol removal, due mainly to the absence of inhibitors.
In the test involving the sample with catalyst (IWW+Catal), a decrease of approximately 12% of total reaction time is observed in relation to the sample without catalyst (IWW) and an increase of only 4% in reaction time in relation to the synthetic sample (SWW). This indicates that somehow, the catalyst is producing an increase in reaction rate, preventing some interference. Although there is still no appropriate mechanism to explain this behavior, it has been assumed that in the process with the catalyst, the ozone-phenol reaction takes place by means other than the simple formation of free radicals. It seems that in this case, the influence of radical inhibitors in the solution is minimal (Lon-gemann, 1997). Thus, the delay shown in relation to the synthetic sample (SWW) is due only to the greater chemical demand of the IWW sample.
The influence of the G/L volumetric ratio is shown in Figure 5. The curves represent the processes carried out on different volumes of industrial wastewater samples. In the first test (IWW ratio G/L 1:2), 20 liters of treated spent caustic are used, and in the second, (IWW ratio G/L 1:3) 30 liters were used. It can be concluded from the diagram that the influence of the volumetric ratio on the reaction system in testing conditions is low. This indicates that the reaction rate is not a determining variable regarding the amount of phenol to be treated because it is very high (K=0.246x lO-3M-1S-1 at pH = 9). Therefore, what limits the reaction rate is added ozone mass and contact system efficiency.
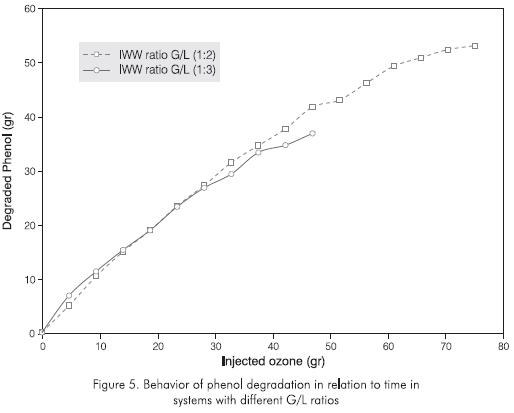
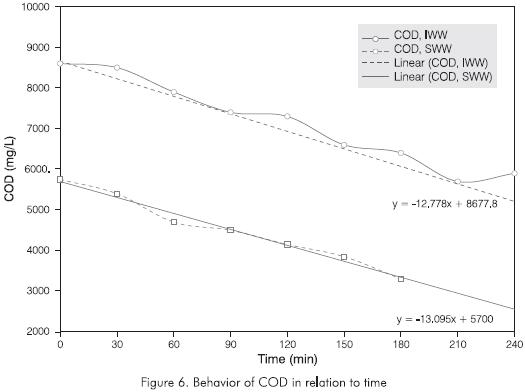
Figure 6 shows the behavior of the COD during the IWW and SWW ozonation tests. It can be observed that COD varies evenly in both cases and with a similar slope, which seems to indicate that the decrease in COD, in reaction conditions, follows a kinetic of first order. However, it is most probable that since the value of this variable is so high, the influence of its decrease on the reaction rate is minimal within the parameters in which the experiment is carried out.
Finally, Figure 7 shows the behavior of the reaction at two different pH values. The curve (pH=10) represents the phenol decrease of industrial wastewater sample (IWW) regarding time with the pH maintained throughout the reaction at 10. The curve (pH=5) shows the degradation of industrial wastewater sample (IWW) under the same conditions but with a pH of five. It can be observed that high pH values increase the oxidation rate of phenol with ozone as has been evaluated previously. In this experiment, the reaction rate for the pH value 10 was approximately 30% higher than for the pH value five, increasing the efficiency for phenol degradation and decreasing ozone consumption by the same order.
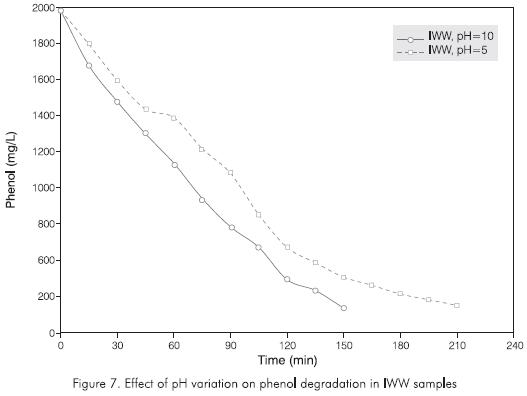
Along with the increase in the reaction rate, there was also an increase in ø ratio, which showed the highest values of all those calculated according to Table 1. This is due mainly to the fact that at low pH, the mechanism of reaction by means of free radicals does not work.
Therefore, what is observed is only the contribution of the direct reaction that impacts the efficiency of the process (Langlais, 1991).
CONCLUSIONS
-
It was proven that the use of ozone as an industrial wastewater pretreatment system is an alternative to be considered in special streams that cannot be treated by biological means due to their toxicity and high content of pollutants, unless they are diluted at a large scale with less toxic streams.
-
The ratio of grams of consumed ozone per grams of degraded phenol in industrial wastewater samples was an average of 1:1.3, and for synthetic water samples was very closed to 1:1 which indicates great reaction efficiency compared to the data reported in literature which show ratios of over 1:2.5.
-
Metallic oxide catalysts increase process rates by decreasing reaction times for the system proposed by approximately 12%.
-
The decrease in pH of the reaction negatively influences the oxidation process of the spent caustic with ozone by increasing reaction times and the ozone consume up to 30%.
-
Ozone treatment of industrial wastewater for some particular cases in the industry, in some streams such as sour waters can facilitate reuse in other processes such as crude desalting, due to the fact that their dilution is avoided and their dissolved solid content is not modified.
-
A more detailed study of the reaction rate in the system and its modeling must be carried out to be able to determine optimum operating parameters, operating under batch or continuous conditions.
REFERENCES
Berné, F. and Cordonnier, I, 1995. "Industrial Water Treatment". Institut Francais du Pétrole Publications, Paris. [ Links ]
Blaich, L., 1999. "Developmenl Of Ozone Technology And Application". Proceedings Of The 14Th Ozone World Congress. Dearbon, MI, Aug. 22-28. [ Links ]
Forero, I., Díaz, I. y Blandón, V, 1999. "Diseño de un nuevo sistema de flotación para el tratamiento de aguas industriales". CT&F-Ciencia, Tecnología y Futuro, 1(5): 67-75. [ Links ]
Forero, I., 2000. "Manejo integral de sodas y disposición de sodas gastadas". Documento interno Ecopeírol, CIT-ICP colección resíringida. [ Links ]
Gurol, M. D. and Singer, P. C, 1982. "Kinetics of Ozone Decomposition". A Dynamic Approach. Envir. Sci. Technol, 21 (895), 16 (7): 377-383. [ Links ]
Gurol, M. D. and Vatista, R., 1987. "Oxidation of phenol by Ozone and Ozone + UV Radiation: A Comparative Study". Wat. Res., 21 (895). [ Links ]
Hoigné, J. and Bader, H., 1977. "Ozonation of Water: Selectivity and Rate of Oxidation of Solutes". Proceedings Of The 3th Ozone World Congress, Paris, France. [ Links ]
Hoigné, J. and Bader, H., 1983. "Rate Constants of Reaction of Ozone with Organic and Inorganic Compounds in Water". Water Research, 17:173-183. [ Links ]
Langlais, B. and Reckhow D. A., 1991. "Ozone in Water Treatment Application and Engineering", Lewis Publishers Inc., Chelsea. [ Links ]
Longemann, F. and Annee, H., 1997. "Water with Fixed Bed Catalytic Ozonation Process". Wat. Sci. Tech., 35, (4): 353-360. [ Links ]
Masten, S. J. and Davies, S. H. R., 1994. "The Use of Ozonation to Degrade Organic Contaminants in Wastewaters". Envir. Sci. Tech., 28 (4): 180A-186A. [ Links ]
Rebhun, M. and Galil, N., 1994. "Technological Strategies for Protecting and Improving the Biological Treatment of Wastewater from a Petrochemical Complex", Wat. Sci. Tech., 29:133-141. [ Links ]
Staehelin, J. and Hoigne, J., 1982. "Decomposition of ozone in water: rate of initiation by hydroxide ions and hydrogen peroxide". Envir. Sci. Tech., 16 (10): 676 - 681. [ Links ]
Trapido, M, 1999. "The Application Of Ozonation and Advanced Oxidation Processes for Degradation and Detoxication of Phenolic Compounds". Proceedings Of The 14th Ozone World Congress, Dearborne, Michigan. [ Links ]














