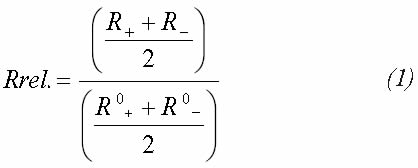Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383On-line version ISSN 2382-4581
C.T.F Cienc. Tecnol. Futuro vol.2 no.4 Bucaramanga Jan./Dec. 2003
BEHAVIOR OF THERMAL SPRAY COATINGS AGAINST HYDROGEN ATTACK
Fabio Vargas*1, Guillermo Latorre*2 and Iván Uribe1
1 Universidad Industrial de Santander, Escuela de Ingeniería Metalúrgica y Ciencia de Materiales
Grupo de Investigación en Corrosión
2 Ecopetrol S.A. - ICP Instituto Colombiano del Petróleo, A.A. 4185 Bucaramanga, Santander, Colombia
e-mail: fabiovargas@citema.org.co e-mail: glatorre@ecopetrol.com.co
(Received 22 July 2003; Accepted 19 November 2003)
*To whom correspondence may be addressed
ABSTRACT
The behavior of nickel and chrome alloys applied as thermal spray coatings to be used as protection against embrittlement by hydrogen is studied.
Coatings were applied on a carbon steel substrate, under conditions that allow obtain different crystalline structures and porosity levels, in order to determine the effect of these variables on the hydrogen permeation kinetics and as a protection means against embrittlement caused this element.
In order to establish behaviors as barriers and protection, hydrogen permeation and corrosion tests under stress were carried out, supported by measurements of the potential’s drop in the substrate-coating system under simultaneous conditions of stress and a corrosive agent with H2S in solution.
Findings show that alloy coatings with face centered cubic structure possess greater hydrogen solubility than coatings with body centered cubic structures and than carbon steel used as substrate. Additionally, porosity inherent to the formation of this type of coating reduces hydrogen diffusion through its structure. The analysis of these results allows selecting the coating with better performance as a protection means against embrittlement by hydrogen.
Keywords: hydrogen embrittlement, thermal spry coatings, hydrogen permeation, corrosion under stress.
RESUMEN
Se estudia el comportamiento de aleaciones de níquel y cromo aplicadas como recubrimientos mediante termorociado, para ser utilizadas como protección ante la fragilización por hidrógeno.
Los recubrimientos fueron aplicados sobre un substrato de acero al carbono, bajo condiciones que permitieron obtener diferentes estructuras cristalinas y niveles de porosidad, con el fin de determinar el efecto de estas variables en la cinética de permeación de hidrógeno y como medio de protección ante la fragilización producida por este elemento.
Para establecer el comportamiento como barrera y protección, se realizaron pruebas de permeación de hidrógeno y de corrosión bajo esfuerzos apoyadas por mediciones de la caida de potencial del sistema substrato recubrimiento en condiciones simultáneas de esfuerzos de tensión y un agente corrosivo con H2S en solución.
Se pudo establecer que los recubrimientos de aleaciones con estructura cristalina cúbica centrada en las caras poseen mayor solubilidad del hidrógeno, que los recubrimientos con estructura cúbica centrada en el cuerpo y que el acero al carbono utilizado como substrato. Adicionalmente, la porosidad propia de la conformación de este tipo de recubrimientos reduce la difusión de hidrógeno a través de su estructura. El análisis de estos resultados permitió seleccionar el recubrimiento de mejor desempeño como medio de protección ante la fragilización por hidrógeno.
Palabras claves: fragilización por hidrógeno, recubrimientos termo-rociados, permeación de hidrógeno, corrosión bajo esfuerzos.
RESUMEN
Estudase o comportamento de ligamentos de níquel e cromo aplicados como recobrimentos mediante termorociado, para serem utilizados como proteção ante a fragilização por hidrogênio.
Os recobrimentos foram aplicados sobre um substrato de aço ao carbono, sob condições que permitiram obter diferentes estruturas cristalinas e níveis de porosidade, com o fim de determinar o efeito destas variáveis na cinética de permeação de hidrogênio e como meio de proteção ante a fragilização produzida por este elemento.
Para estabelecer o comportamento como barreira e proteção, realizaramse provas de permeação de hidrogênio e de corrosão sob esforços apoiados por medições da queda de potencial do sistema substrato recobrimento em condições simultâneas de esforços de tensão e um agente corrosivo com H2S em solução.
Pôdese estabelecer que os recobrimentos de ligas com estrutura cristalina cúbica centrada nas faces possuem maior solubilidade do hidrogênio, que os recobrimentos com estrutura cúbica centrada no corpo e que o aço ao carbono utilizado como substrato. Adicionalmente, a porosidade própria da conformação deste tipo de recobrimentos reduz a difusão de hidrogênio através da sua estrutura. A análise destes resultados permitiu selecionar o recobrimento de melhor desempenho como meio de proteção ante a fragilização por hidrogênio.
INTRODUCTION
Damages caused by hydrogen on steel are a frequent problem in the oil industry and usually compromise operational integrity, especially of equipment used in hydrocarbon refinement, thus generating high maintenance costs.
Several equipment used in oil refinement process with H2S in solution of up to 50 ppm or more, making it susceptible to Hydrogen-Induced-Cracking (HIC), Stress-Oriented Hydrogen Induced Cracking (SOHIC), Sulfur Stress Cracking (SSC), and blistering.
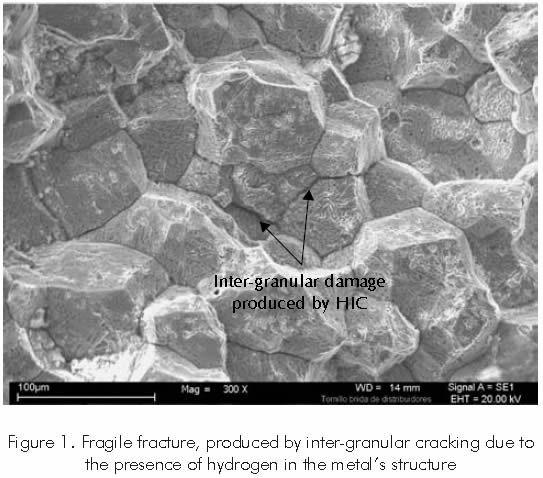
One of the most critical damages is induced cracking (HIC), produced in high resistance and low alloy steel, due to the absorption, trapping, and recombination of atomic hydrogen in the internal structure of the material (Dias, 1994; Fukai, 1993; Hirth, 1984). Such trapping might imply an increment in molecular hydrogen pressure that, together with the effects of tensions and discontinuities concentration on the steel structure, originate fragile cracking process such as that shown in Figure 1, where the morphology of the inter-granular damage induced by the presence of hydrogen in the structure of a component of the Barracabermeja refinery can be observed.
Costs involved in unscheduled stops, equipment repairs, potential risks for workers, and deterioration of the environment caused by HIC, justify the research efforts carried out to understand the mechanisms inherent to the occurrence of this cracking phenomenon, to establish reliable lab tests that reproduce conditions at which this type of damage is produced, and to search for alternate solutions.
Application of alloy coatings resistant to cracking produced by hydrogen on metallic substrates of lower cost (for example, carbon steel) is a technically and economically favorable alternative for the solution of these problems. Thermal-spray is a relatively new technique that enables the application of coatings of diverse alloys to components and equipment of different geometries and sizes, both at plant and field levels.
Due to the characteristics inherent to the application process, thermal-spray coatings feature pores that, under certain conditions, may act as traps to stop hydrogen that has been able to enter the coating structure (Iwona and Karol, 1998; Herman and Sampath, 1996).
Nevertheless, it is important to emphasize that the final performance of the thermal-spray coatings used as a barrier to block the hydrogen path depend on the chemical composition of the material used as coating, its microstructure, its structural defects, and its capacity to keep its structural integrity under stress conditions to which it might be exposed during use.
EXPERIMENTAL DEVELOPEMENT
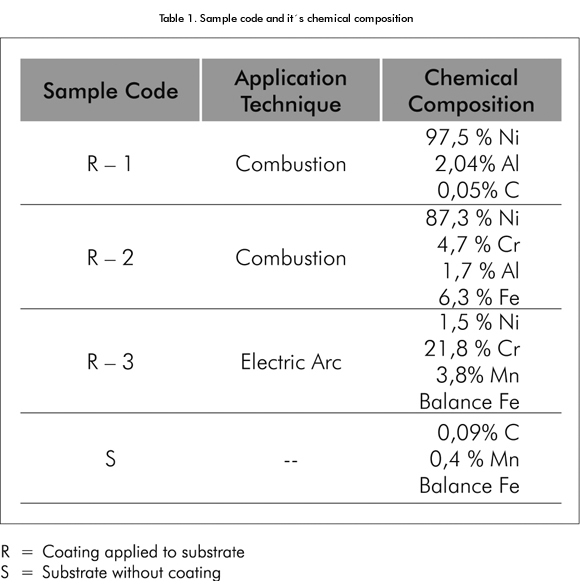
For the execution of these tests, samples were prepared through the application of alloy coatings with high contents of nickel and alloys with high contents of chrome, using combustion and electric arc techniques on carbon steel AISI 1010 substrates. The samples were coded according to the process performed, as shown in Table 1.
The coatings applied through combustion thermal spray used metallic powders heaters with a flame produced with a mixture of acetylene - oxygen, while the arc spray coatings was applied heating a metallic wire with electric arc.
Chemical characterization
The chemical composition of the alloys applied as coatings in samples R-1 and R-3 was determined by Optical Emission Spectrometry (OES). As for sample R-2, it was necessary to apply the Atomic Absorption (AA) technique, since verification patterns through spectrometry for this alloy are unavailable.
Structural and microstructural analysis
The analysis of the microstructure and defects on the applied coatings was carried out through optical microscopy, complemented with a digital image analysis sotfware to identify and quantify the presence of pores. The analysis was carried out on the transversal section of each of the applied coatings, revealing the phases present by chemical attack.
Hydrogen permeation
The kinetics study of hydrogen permeation in carbon steel samples coated with different alloys was carried out according to ASTM G148-97 standard, through Devanathan’s duplo-potentiostatic technique, complemented by the methodology developed for the study of hydrogen permeation in coated substrates (Miranda and Fassini, 1993), which establishes that the current variation generated by hydrogen diffusion in the sample through time shall be measured both in the coated face and in the substrate facing the hydrogen generating environment in the charge cell.
As for experimental traceability, letters (RS) or (SR) are added to the samples to be evaluated, according to the arrangement of the samples in the test, in such a way that the (RS) code indicates that the sample was placed with the coated face facing the hydrogen generating environment in the charge cell. The (SR) code indicates that during the test, the substrate was placed with the substrate facing the hydrogen generating environment in the charge cell.
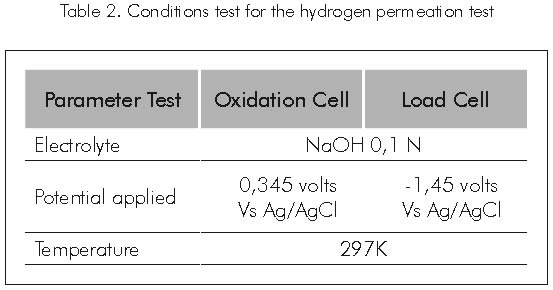
Figure 2 shows Devanathan’s cell, as used to perform the hydrogen permeation tests. The test conditions under which the hydrogen permeation were carried out are shown in Table 2.
Corrosion under stress test
Corrosion test under stress test was performed according to the NACE TM 0177-96 standard, to evaluate the behavior of the studied coatings under simultaneous conditions of tensile stress and highly hydrogenated corrosive environment.
Intended to be used as a corrosive medium, an aqueous solution of pH 2,99 was prepared with 5% in weight of NaCl, 1*10-3 mol/liter of hydrated sodium thiosulfate Na2S2O3. 5 H2O and 0,5% of acetic acid. This allows achieving a high content of H2S, one of the main promoters of embrittlement by hydrogen. In the cell used to carry out this test, a potential of -600 mV was applied between the auxiliary platinum electrode and the sample test, to produce H2S in solution through electrolysis.
To verify the integrity of the coating during the test and its effectiveness as a protection medium for the substrate against embrittlement by hydrogen, this was periodically monitored by means the potential drop technique, which, according to Tsai and Shih (1997), is adequate for such purpose. This test involves the application of a current of 500 mA to the sample immersed in the solution test and to a reference sample in air, and the measurement of the response voltage. The resistance of the sample may be calculated through Ohm’s law.
Afterwards, the current is inverted to -500 mA and the new value for the resistance is calculated. Relative resistance for the sample in air and that of the test in the aqueous solution (with H2S in solution) was calculated through the following expression (Tsai and Shih, 1997):
R+ is the resistance of the sample in the corroding medium whenever a positive current is applied
R0+ is the resistance of the sample in air whenever a positive current is applied
A change in the system’s relative resistance is an indicator of deterioration of the sample that acts as a conducting medium of the current fed into the circuit.
The tensile stress applied to each of the samples test was 80% of the elastic limit for the material used as substrate.
Effectiveness of the coatings studied as a protective medium against embrittlement by hydrogen was analyzed through the elongation suffered by the assayed sample prior to the fracture and through the relative resistance of the coating-substrate system.
RESULTS
Structural and microstructural analysis
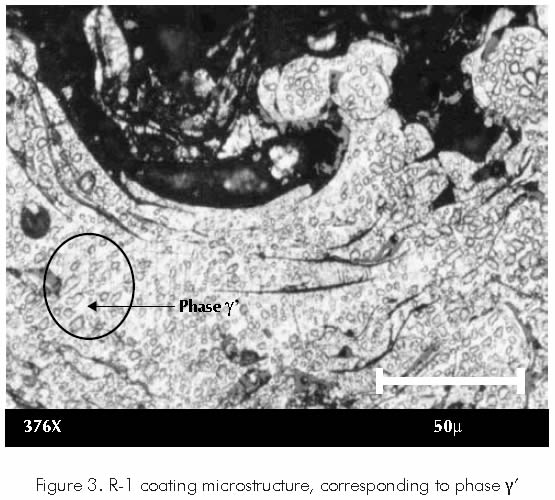
Micro-structural and structural analysis carried out through optical microscopy to each one of the samples showed that coating R-1 features high contents of a nickel-rich phase, known as gamma prima (γ’), which has a face centered cubic structure, being one of the most compact structures to be found in metals. Figure 3.
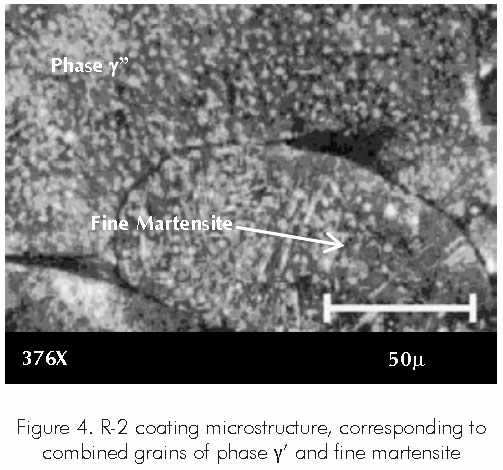
Coating R-2 contains grains with combined phases corresponding to a gamma prima (γ’) and fine martensite. Martensite possesses a body centered cubic structure, being one of the least compact structures to be found in metals. Figure 4.
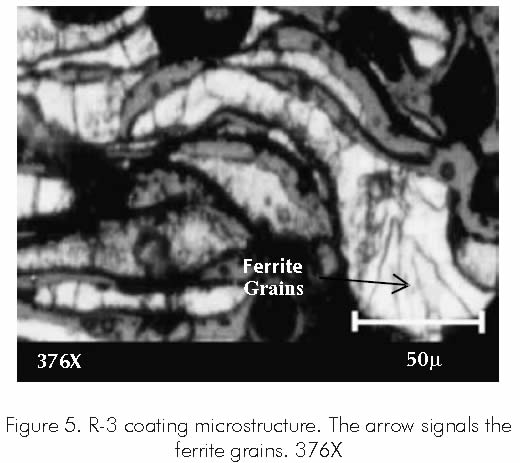
Coating R-3 features a microstructure with high content of ferritical phase, with body centered cubic structure, being one of the least compact structures to be found in metals. Figure 5.
The percentage of pores found in each of the samples analyzed was: R-1=18,96±1,4; R-2=5,27±0,74; R-3=7,43±0,6.
Hydrogen permeation
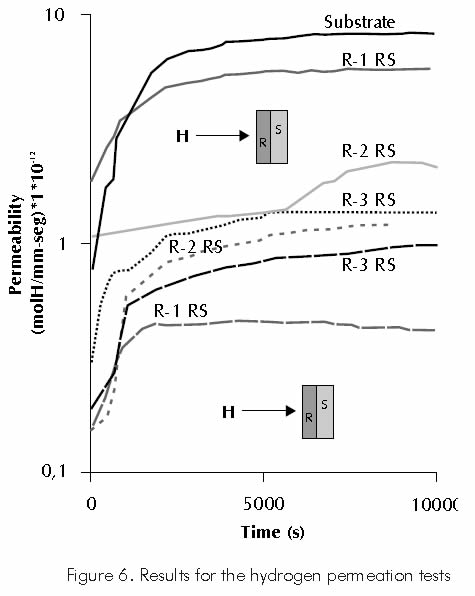
Each of the samples assayed presented different current variation, produced by hydrogen diffusion in the sample through time. Based on the results for each of the tests carried out, hydrogen permeation curves were constructed as shown in Figure 6.
To determine the influence of structural defects in the hydrogen permeation kinetics, each of the studied samples was subject to a second permeation test, in which the sample is set with the coated face facing the hydrogen generating environment in the charge cell.
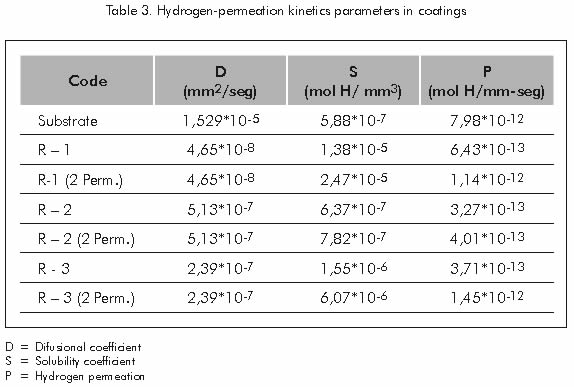
Table 3 reports the parameters of the hydrogen permeation kinetics as calculated from the results obtained in the first and second hydrogen permeation carried out on the studied samples.
Corrosion under stress test
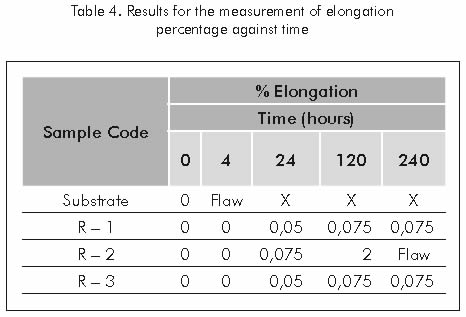
Each of the samples evaluated through the corrosion under stress test, showed a different elongation percentages prior to the fracture and different levels of coating deterioration through time.
The elongation percentage experimented by each sample prior to fracture and the time at which the fault occurred are reported in Table 4.
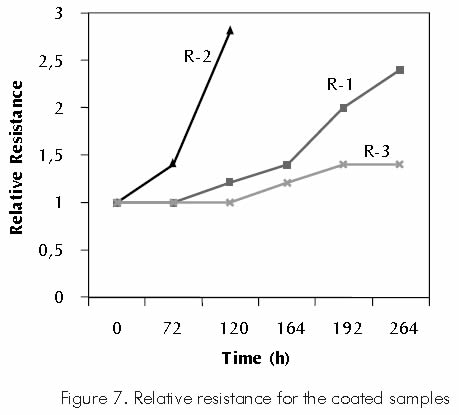
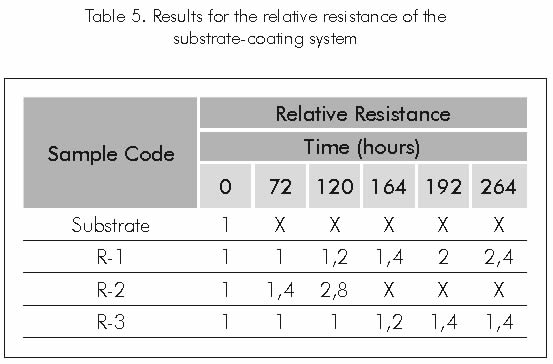
Results for the relative resistance produced by the coating’s deterioration (break up) and calculated as the voltage measures found in the potential fall test, are shown in Table 5 and represented in Figure 7.
ANALYSIS AND DISCUSSION OF RESULTS
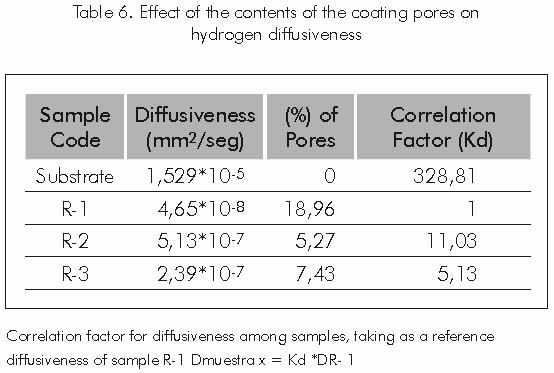
Hydrogen diffusivity calculated for each coating applied to the substrate, decrease as the percentage of pores that contain these coatings increases. See Table 6.
Table 6 shows that hydrogen diffusivity in carbon steel used as substrate is 328,81 times greater than the diffusivity of this element in sample R-1, which features the largest content of pores and therefore, shows the lowest hydrogen diffusivity rating. Reduction in hydrogen diffusivity in coatings with a high content of pores may be attributed to the behavior of these empty spaces, acting as a proper site for the recombination of atomic hydrogen where molecules and hydrogen compounds may be formed, remaining trapped in these places.
Iwona and Karol (1998), established that coatings applied by thermal-spray may impede the effective diffusion of hydrogen through its structure, by trapping hydrogen atoms in interfaces or other structural imperfections in these type of coatings.
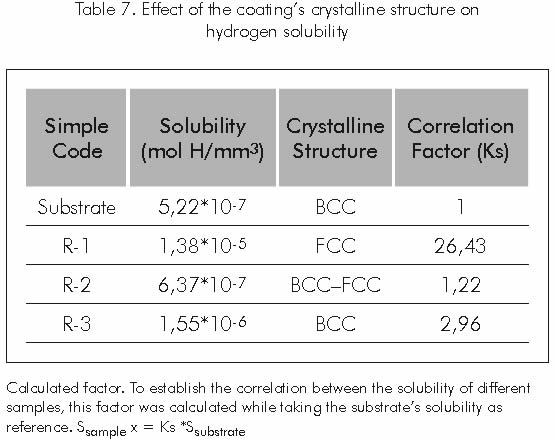
Hydrogen’s greatest solubility was found in the coating of sample R-1, which features high precipitation of gamma prima Γ’ phase. This behavior may be attributed to the high solubility that hydrogen has in the face centered cubic structure, typical of this phase (Silva et al., 1984).
Table 7 shows that hydrogen solubility in the face centered cubic structure in the coating of sample R-1 is 26,43 times greater than the solubility of this element in the body centered cubic structure of carbon steel used as substrate and 8,9 times greater than the one in the R-3 sample’s coating.
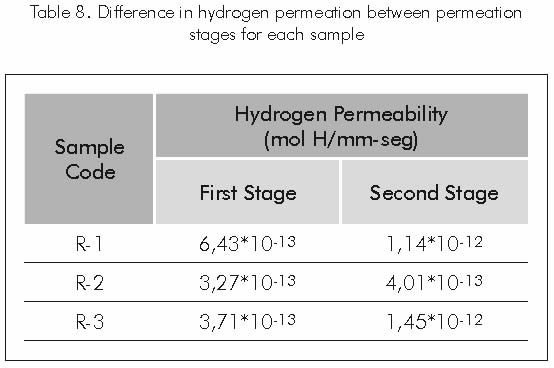
Increases in hydrogen permeability during the second permeation of each coating applied to the substrate may be attributed to the partial or total filling with hydrogen of the pores or empty spaces in the coating that during the first permeation probably acted as a hydrogen retention medium; therefore, during the second permeation, hydrogen that enters the coating structure goes through it with less difficulty, since part of the traps (pores) are filled with molecules or hydrogen compounds. Table 8 shows the difference between the first and second stages of hydrogen permeation for each sample.
During the corrosion under stress test, samples coated with alloys featuring high nickel contents (97, 5 % Ni; 87, 3% Ni) and high chrome contents (21,8% Cr), presented higher elongation ratings prior to fracture, as compared against the uncoated sample; therefore, protection given by these coatings to the substrate against the harmful effects of the H2S contained in the Sodium Thiosulfate solution, retards nucleation and unstable propagation of cracks.
Failure by the sample coated with coating R-2 after 240 hours of test is probably due to the synergy caused by the tensile stress applied to the sample test and to embrittlement by hydrogen that the martensitical particles may suffer.
Coating R-1 sample shows a high tendency to grow in relative resistance through time, indicating progressive deterioration of this coating. This deterioration is caused by the combined effect of possible blistering in the layer’s interior and the tensile stress applied during the test.
Blistering in thermal-sprayed coatings is generally due to excessive retention and recombination of hydrogen inside the pores or empty spaces.
KNOWLEDGEMENTS
The authors wish to express their most sincere acknowledgement to the Materials Technology Area at the Instituto Colombiano del Petróleo (ICP), and to the Corrosion Research Group at the Universidad Industrial de Santander, for their valuable collaboration and support.
BIBLIOGRAPHY
Dias, M. F. D., 1994. "Estudo dos efeitos da fragilizaçao pelo hidrogênio nos aços UNS - G41300E UNS - S 31803 através de ensaios de traçao con baixa taxa de deformaçao en soluçoes de tiossulfato de sodio". Universidade Federal Do Rio de Janeiro. [ Links ]
Fukai, Y., 1993. "The metals hydrogen system". Springer-Verlag, New York. [ Links ]
Herman, H. and Sampath, S., 1996. "Thermal spray coatings". In: Stern, K. H., Ed., Metallurgical and Protective Coatings, 1 Ed., Chapter 10, New York, Chapman & Hall. [ Links ]
Hirth, J. P., 1984. "Thories of hydrogen induced cracking of steels". Hydrogen Embrittlement and Stress Corrosion Cracking, A.M.S., Editorial R. Gibala y R. F. Hehemann, 29-4. [ Links ]
Iwona, S. O. and Karol, E. S., 1998. "Developing a methodology for performance evaluation of metallic thermal spray coating for oil and gas industry service". Materials Performance, July, 37 (7): 34-40. [ Links ]
Miranda, P. E. and Fassini F. D., 1993. "New methodology for the determination of hydrogen permeation parameters in layered materials". J. Mater. Scien., 28: 5148-5154. [ Links ]
Miranda, P. E., 1984. "Hydrogen induced surface effects on the mechanical properties of type 304 stainless steel". In: Fracture Prevention in Energy and Transport Systems, Le May e S. N., Monteiro ed., London, EMAS, 511-520. [ Links ]
Silva,T. C., Pascual, R. and Miranda, P. E., 1984. "Hydrogen induced surface effects on the mechanical properties of type 304 stainless steel ". In: Fracture Prevention in Energy and Transport Systems, Le May e S. N. Monteiro ed., London, EMAS, 511-520. [ Links ]
Tsai, S. Y. and Shih, H. C., 1997. "The use of thermal spray coatings for preventing wet H2S cracking in HSLA steels plates". Corrosion Prevention and Control, 42-48. [ Links ]