Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383On-line version ISSN 2382-4581
C.T.F Cienc. Tecnol. Futuro vol.3 no.2 Bucaramanga Jan./Dec. 2006
Marcio V. Reboucas1, Ana-Rosa C. G. Massa1, and Marcos Vinicio Assis1
1 Braskem S.A., Unidade de Insumos Basicos, Laboratorio, Rua Eteno 1561, Complexo Petroquimico de Camacari, Camacari-Bahia, Brazil, 42810-000. e-mail: marcio.reboucas@braskem.com.br
(Received May 14, 2006; Accepted Oct. 19, 2006)
ABSTRACT. Potentiometric titration using silver nitrate for determination of sulfide and mercaptide ions, as defined by the UOP Method 209, has been widely used for quality control of Spent Caustic Neutralization Unit in petroleum industries. However, it has been found this procedure does not provide accurate results for samples taken from units based on Wet Air Oxidation (WAO) technology. Therefore, possible sources of error, such as concominant species, between-analytes interference and titration solvent, were investigated and adjustments in the method were proposed. Aqueous media should be used to prepare the titration solution instead of the recommended alcoholic solvent which was found to be related to the quite low analyte recoveries in WAO unit samples. The modified method can be successfully applied to samples with different chemical compositions which is the main advantage over the reference procedure. Limit of detection and relative standard deviation of 0,5 mg.L-1 and 5,2% for sulfide and 0,6 mg.L-1 and 5,5% for mercaptide were achieved.
Keywords: sulfide, thiol, potentiometric titration, interference, sodium hydroxide.
RESUMEN. La titulación potenciométrica utilizando nitrato de plata para determinación de iones de sulfuro y mercaptido como lo define la UOP Método 209, ha sido ampliamente utilizada para establecer el control de calidad de la Unidad de Neutralización Cáustica para las industrias del petróleo. Sin embargo, se ha establecido que este procedimiento no arroja resultados precisos para muestras tomadas de unidades basadas en Oxidación por Aire Humedecido (WAO). Por consiguiente, se investigaron las fuentes de error tales como especies concomitantes, interferencia entre análitos y solvente de titulación y se propusieron ajustes del método. Deben utilizarse medios acuosos para preparar la solución de titulación, en lugar del solvente alcohólico recomendado, el cual demostró estar relacionado con las recuperaciones de análitos bastante bajas en las muestras de la unidad WAO. El método modificado puede aplicarse con éxito a muestras que tengan distintas composiciones químicas, lo cual es la principal ventaja sobre el procedimiento de referencia. Se lograron límites de detección y desviaciones estándar relativas de 0,5 mg.L-1 y 5,2% para sulfuros y 0,6 mg.L-1 y 5,5% para mercaptidos.
Palabras clave: sulfuro, thiol, titulación potenciométrica, interferencia, hidróxido de sodio.
INTRODUCTION
Spent caustic stream is originated in the process of ethylene production at petrochemical plants. This stream derives from caustic washing of ethylene which aims at removing acid gases, such as hydrogen sulfide, from the final product. The spent caustic contains large amounts of hydrogen sulfide, some mercaptans and emulsified hydrocarbons. Therefore, to be sent to an Effluent Treatment Unit for further treatment before final disposal, the spent caustic stream should be submitted to a process in which its pH is adjusted and most of the sulfide (S2-) and mercaptide (RS-) ions are removed.
There are two basic processes to neutralise the spent caustic, although other treatment processes are also available (Sheu & Wen, 2001). The first one is based on Direct Acid Neutralization (DAN) with sulfuric acid, followed by steam stripping (Carlos & Maugans, 2002; Sheu & Weng, 2001). Sulfide and mercaptide ions are oxidised to sulfate ions and residual hydrogen sulfide and volatile mercaptans are removed by stripping the neutralised solution.
The second process uses a more modern technology named Wet Air Oxidation (WAO) (Luck, 1999). In such process, almost all sulfide ions are oxidised to thiosulfate and, in a lesser extent, sulfate ions. The expected concentration of thiosulfate and sulfate ions in the neutralised solution are 10,000 and 2,000 mg.L-1 respectively. Mercaptans are converted to dissulfide organic compounds which tend to migrate to oil phase due to their higher molecular weight, and hence are removed from spent caustic solution. The oxidised solution is neutralised with sulfuric acid and its pH adjusted from 13 to 6-9.
Therefore, the final effluent from the Spent Caustic Neutralization Unit should be continuously monitored regarding its pH and sulfide and mercaptide ions content. Most refineries and petrochemical plants over the world employs a quite standardised procedure based on potentiometric titration established by UOP Method 209. The method consists of measuring the potencial difference during sample titration with an alcoholic solution of 0,01M silver nitrate. Silver ion reacts quantitatively with sulfide and mercaptide ions, according to the reactions given below:
S2- + 2 Ag+ ? Ag2S ? (black precipitate)
RS- + Ag+ ? RSAg ? (yellow precipitate)
The samples are titrated in 1M sodium hydroxide to which ammonium hydroxide is added to prevent precipitation of silver oxide (Tamele, Ryland, & McCoy, 1960). When all content of the analytes has been consumed, the excess of silver ion present in solution causes an abrupt potencial difference which is measured by the silver electrode and determines the final point of the titration.
However, limited information is available on the literature regarding interferents in the analysis and the suitability of the method to an effluent originated from new units based on more modern technologies. In fact, the more relevant papers on this subject date from the 50´s and 60´s. Tamele et al. (1960) suggested most of species normally encountered in petroleum processing did not interfere. Certain anions such as iodide, bromide, and cyanide interfere by forming very insoluble silver salts, but they are not expected to be present in the spent caustic samples. Some strongly reducing species also interfere by reducing the silver titrant to metalic silver; this interference is evidenced by a rapid darkening of the solution and formation of a silver mirror on the walls of the titration beaker. Karchmer (1957) also pointed out possible interference caused by inorganic polysulfides (RSS)- whose formation during titration is related to the presence of elemental sulfur and certain mercaptan species. However, Karchmer (1958) also suggests elemental sulfur is unlikely to be present in aqueous samples.
As previously mentioned, the composition of an effluent from a WAO Unit is expected to be quite different to the one obtained from a DAN Unit. As a result, our team has experienced serious problems in obtaining accurate and precise results when using the original method to analyse WAO Unit effluents. It is worthy pointing out that, besides sulfate and thiosulfate, none of the possible interferents mentioned before were detected to justify the unsatisfactory results.
Therefore, the present work aims at improving the method accuracy by studying the effect of possible interferents such as sulfate and thiosulfate ions, the influence of the sulfide concentration in the mercaptide determination and vice-versa, and propose modifications in the original method to guarantee its suitable application to samples obtained from different units.
EXPERIMENTAL
Reagents and materials
All reagents used were of analytical grade and free of dissolved air and peroxidic impurities to prevent losses of the easily oxidised sulfur compounds. Deionised water was obtained from a Mili-Q Reagent-grade Water System (Milipore), resistivity 18 M?.cm-1.
Concentrated NH4OH and NaOH were used to prepare the diluted required solutions.
The titrant solution (0,01M silver nitrate aqueous solution) was prepared from AgNO3 salt, minimum purity 99,8%m/m. To obtain the titration solvent, a 50mL aliquot of 1M NH4OH was added to 1000mL of 1M NaOH.
Sulfide and mercaptide standard solutions were daily prepared from (NH4)2S minimum purity 20%m/m e C2H5SH minimum purity 97%m/m in 5M NaOH solution. Sulfate and thiosulfate solutions were prepared from Na2S2O3.5H2O minimum purity 99,5%m/m and Na2SO4 minimum purity 99,9%m/m in 5M NaOH solution.
Apparatus
All experiments were carried out in an automatic potentiometric titrator (Metrohm, model 716 DMS) equipped with magnetic stirrer (Metrohm, model 649) connected through a RS232 interface to an Intel PC. A combined electrode Ag/Ag2S (Metrohm, Switzerland) was used. The electrode was rinsed with water after each analysis and reconditioned on weekly basis through the procedure described in UOP-209 Method. The data was recorded and the graphs were displayed using an acquisition software developed in our own lab.
Procedure
An aliquot of the caustic sample is weighed and transferred to the titration beaker. The sample size should be selected, usually in the range from 0,1 to 10g, to give a titration volume of at least 2mL. Approximately 100mL of the titration solvent is added to the sample. The beaker is connected to a nitrogen supply since blanketing with an inert gas prevents the analytes in the sample from air oxidation. The mixture is titrated with 0,01M aqueous silver nitrate at 0,05mL increments while stirring at a moderate speed. The end point of the titration is given by an abrupt voltage difference which occurs when the electrode detects excess of silver ions in solution. If both sulfide and mercaptide ions are present, two inflexions are observed. The first derivative is often used to help to determine the end point(s). The aqueous solvent for the titration solution replaced the alcoholic media (2-propanol) recommended by UOP Method 209.
RESULTS AND DISCUSSION
Evaluation of titration curves
The potentiometric curves for three standards containing sulfide, mercaptide and both sulfide and mercaptide ions are shown in Figure 1.
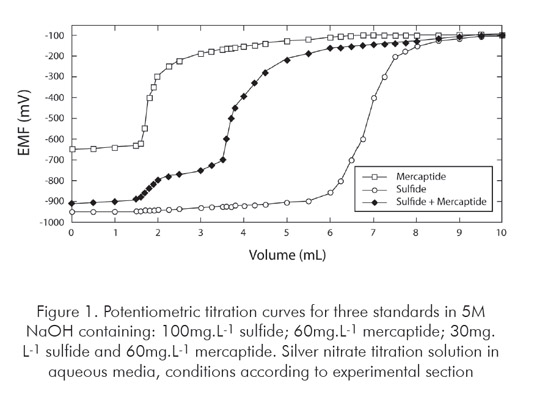
When only sulfide is present, the inflexion begins at about –950 mV; if only mercaptide is present in solution, the voltage begins to change around –650 mV. If both sulfide and mercaptide are present, the behaviour remains quite similar in terms of beginning and ending of curve inflexion, and hence two inflections will be noted in titration curve. The first one is related to the presence of sulfide ions while the second one to mercaptide ions. If none species is present, the measured voltage difference at the beginning of titration is near –200 mV and remains at this range until the end of titration.
It is worthy noticing the shape of the titration curve is strongly dependent of some factors such as concentration and type of analyte species, relative concentration between the analytes, sample mass taken to analysis and sample matrix. Such factors cause the inflexions become closer to each other or become less abrupt, for example.
When only one inflexion is present, the UOP method recommends confirming the identity of the species using a lead acetate test paper. This recommendation may be followed but a straightforward evaluation of the titration curve is sufficient to identify the species present in solution, as previously described. Therefore, such interpretation of the titration results avoids the need of additional tests and speeds up the analysis.
Effect of sulfate and thiosulfate ions
Once sulfate and thiosulfate are present in much higher concentrations than the analytes, the interference of such ions was investigated since no similar data was found in the literature. Increasing concentrations of the concomitants were added to a standard solution and the results of sulfide and mercaptide were recorded. Sulfate was tested to a maximum concentration of 6 000 mg.L-1, while thiosulfate was added to reach a maximum concentration of 100 000 mg.L-1. The data obtained in such experiments are shown in Figure 2.
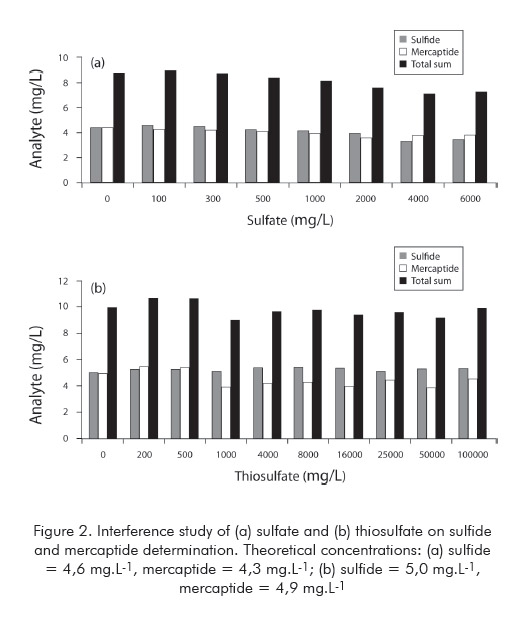
Neither sulfate nor thiosulfate showed a significative interference effect. Above 2 000 mg.L-1 of sulfate the sum of both analytes showed a slight drop from the theoretical value. However, this corresponds to a concentration decrease of less than 10% for each analyte. The presence of thiosulfate caused a discreet change in the distribution between sulfide and mercaptide above 500 mg.L-1, although the sum of the analytes remained steady over the thiosulfate concentration range. The effect on mercaptide results due to higher thiosulfate concentrations is statistically significative since it is above the observed repeatability of the proposed method (ca. 0,7 mg L-1S2- and RS- for the concentration level used in this study section). However, even this effect do not represent a relevant impact on the analytical results in practical terms.
Effect of the ratio between the analytes
To a sulfide standard solution were added increasing amounts of mercaptide ion while the sulfide result was recorded. A similar approach was applied using a mercaptide standard to assess the effect of excess of sulfide. The results are shown in Figure 3.
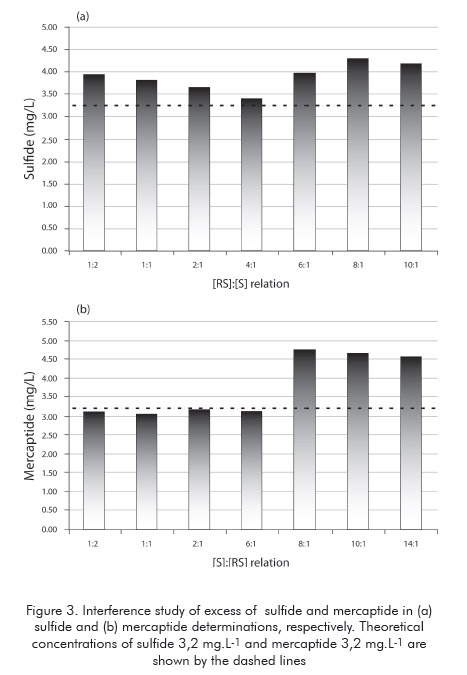
The results demonstrated there is no marked influence of mercaptide excess on the sulfide determination in the tested range. On the other hand, the presence of excess of sulfide to mercaptide, in a ratio equal or greater than eight, causes a significative increase on the mercaptide results. The UOP method mentions such an interference, although it states this effect is observed only if the ratio of sulfide to mercaptide is greater than 10. These conclusions are in accordance with the repeatability values observed in the concentration level used in this study (ca. 0,6 mg L-1S2- and RS- ).
Effect of the solvent of the titration solution
Although all considerations presented so far had been taken into account, it was observed a pronounced interference in the analytes determination in samples taken from WAO Unit. This conclusion is readily drawn from the analytical results using alcoholic titration solution shown in Figure 4, which brings the recovery factors from analyte additions in real WAO effluent samples. No interference has been noticed in samples from DAN Unit.
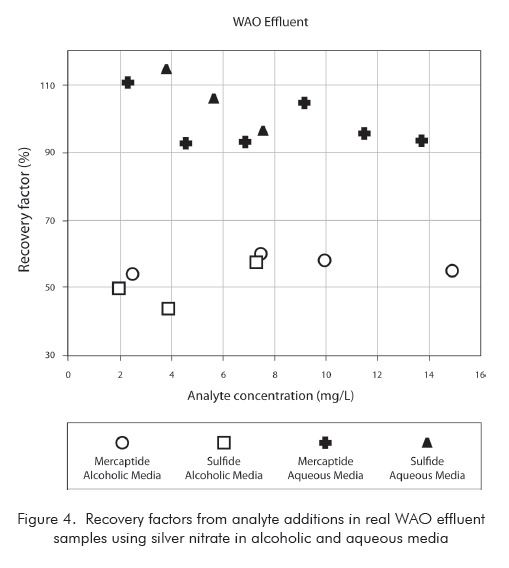
Therefore, it was investigated the effect of changing the solvent used to prepare the silver nitrate titration solution. According to UOP method, the solution should be prepared in alcoholic media, using 2-propanol. However, according to some few references (Tamele et al., 1960, & Karchmer, 1958) the alcoholic solvent was formerly applied to analysis of petroleum products to help their solubilization. Therefore, an aqueous titration solvent was prepared and tested using analyte addition to real WAO samples, as shown in Figure 4. As the results with aqueous titrant showed good recovery factors from analyses, this modification was proposed and validated.
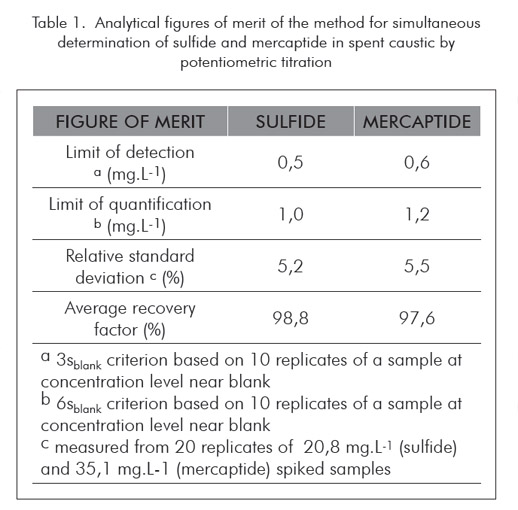
Method validation
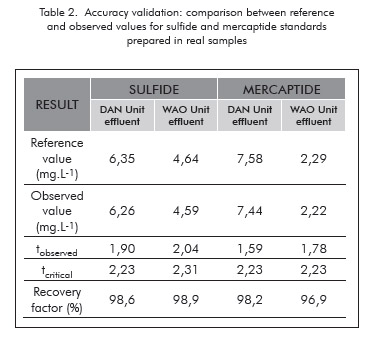
The method was validated following well established statistical procedures. The figures of merit of the validated method are summarized in Table 1. Accuracy was evaluated comparing the observed results of a standard to the theoretical ones through t-test. Each standard was prepared using samples taken from WAO and DAN units, after acidification and purging until complete removal of the analytes, which was confirmed by analysis. The average of ten replicates was compared to the reference value for each analyte as shown in Table 2. Since the tobserved values were always below the tcritical values, one cannot reject the null hypothesis the obtained average and reference value come from the same population or, in other words, there is no significative statistical difference between the analytical result and theoretical value.
Precision was assessed through analysis of spiked samples by four different technicians in five replicates each. The overall relative standard deviations (RSD) obtained from the 20 replicates were found to be below the maximum acceptable RSD (5,9%) as defined by the Horwitz (1982) criterion at the concentration level used.
The limits of detection and quantification observed were 0,5 mg.L-1 and 1.0 mg.L-1 for sulfide and 0,6 mg.L-1 and 1,2 mg.L-1 for mercaptide, respectively.
Sample analysis
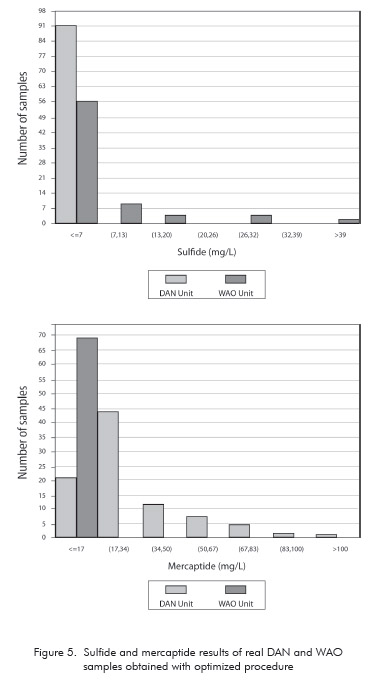
The method has been applied to analysis of effluents from WAO and DAN Units. Figure 5 presents the histograms of the analytical results of 170 samples collected over a period of one month. The data show a quite different distribution profile of sulfide and mercaptide between DAN and WAO Units. The concentration of sulfide in all samples taken from DAN Unit is below 7 mg.L-1, while some higher values can be observed in WAO samples. On the other hand, mercaptide concentration in DAN effluents is often higher than in WAO Units. Such behaviour results from differences in processing conditions and also in the feed composition of each unit.
CONCLUSIONS
Relevant differences between the UOP reference method and the proposed method regarding interferences and data accuracy have been described.
It was proved that aqueous media should be used to prepare the titration solution instead of the recommended alcoholic solvent which was found to be related to the quite low analyte recoveries in samples from WAO units.
The presence of excess of sulfide to mercaptide in a ratio equal or greater than eight causes a significative increase in the mercaptide results, differently of the ratio of 10 stated in the UOP method. Excess of mercaptide to sulfide does not show any significative effect on sulfide determination. Sulfate and thiosulfate ions do not show a significative interference effect at the concentration levels usually found in spent caustic samples.
The proposed method was demonstrated to be accurate and precise. Limits of detection and quantification of 0,5 mg.L-1 and 1,0 mg.L-1 for sulfide and 0,6 mg.L-1 and 1,2 mg.L-1 for mercaptide, respectively, were achieved. The relative standard deviations were found to be 5,2% for sulfide at 20 mg.L-1 and 5,5% for mercaptide at 35 mg.L-1 level.
ACKNOWLEDGEMENTS
The authors thank Braskem S.A. for supplying technical resources required for this work and Professor Antonio Celso Spinola for helping to find a key reference cited in this work.
REFERENCES
Carlos, T. M. S., & Maugans C. B. (2002). Manage refinery spent caustic efficiently. Hydrocarbon Processing, 81 (2), 89-92. [ Links ]
Horwitz, W. (1982). Evaluation of analytical methods used for regulation of food and drugs. Anal. Chem., 54: 67A-76A. [ Links ]
Karchmer, J.H. (1957). Potentiometric determination of mercaptans in presence of elemental sulfur, Anal. Chem., 29: 425. [ Links ]
Karchmer, J.H. (1958). Determining sulfur compounds in petroleum naphtha – The humble scheme. Anal. Chem., 30: 80. [ Links ]
Luck, F. (1999). Wet air oxidation: past, present and future. Catalysis Today, 53: 81-91. [ Links ]
Sheu, S., & Weng, H. (2001). Treatment of olefin plant spent caustic by combination of neutralization and fenton reaction. Wat. Res., 35: 2017. [ Links ]
Tamele, M.W., Ryland, L.B., & McCoy, R.N. (1960). Simultaneous determination of hydrogen sulfide and mercaptans by potentiometric titration. Anal. Chem., 32: 1007. [ Links ]
UOP Method 209-00. Alkalinity, sulfide and mercaptide analyses of used refinery caustic solutions. [ Links ]














