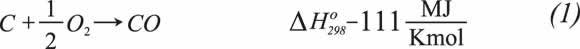Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383On-line version ISSN 2382-4581
C.T.F Cienc. Tecnol. Futuro vol.3 no.5 Bucaramanga Jan./Dec. 2009
SYNGAS OBTAINMENT FROM THE GASIFICATION OF ASPHALTENES OF THE SAN FERNANDO CRUDE OIL
Laura-Smith Moreno-Arciniegas2*, Fabio-Ernesto Rodríguez-Corredor2*, Luz-Edelmira Afanador-Rey1* and Jorge-Luis Grosso-Vargas2
1 Ecopetrol S.A-Instituto Colombiano del Petróleo, P. O. Box 4185, Bucaramanga, Santander, Colombia
2 Universidad Industrial de Santander (UIS), Bucaramanga, Santander, Colombia
e-mail: luze.afanador@ecopetrol.com.co e-mail: laurasmith.moreno@gmail.com
e-mail: fabio.rodriguez.corredor@gmail.com
(Received April 30, 2008; Accepted July 17, 2009)
* To whom correspondence may be addressed
ABSTRACT
In this work, we developed the first study in Colombia to obtain and evaluate syngas compositions derived from asphaltenes gasification. These asphaltenes came from the implementation of a Deasphalting process to San Fernando crude oil, with the purpose of looking for technological options for their utilization. We performed the design, installation and commissioning of facilities for the gasification of asphaltenes at laboratory scale, it following an experimental methodology, performing nine tests and considering temperature and agent gasification quantity (oxygen) as independent variables. The syngas derived from gasification was analyzed by two chromatographic techniques, which reported the presence of refinery gases and sulfur. We evidenced a growth tendency of CO, H2 and sulfur composition and a decrease in CH4 and CO2 composition with temperature. The composition of the syngas was evaluated with different quantities of gasification agent (33%, 40% and 47% the amount of oxygen theoretically required for complete combustion) at each temperature levels operated. It was established that when using a 40% of gasification agent, you get greater average content of CO and H2, which are the interest gases in the gasification process.
Keywords: San Fernando crude oil, gasification, asphaltenes, syngas.
RESUMEN
En este trabajo se desarrolló el primer estudio en el país de la obtención y evaluación de las composiciones del syngas a partir de la gasificación de asfaltenos provenientes de la aplicación del proceso de Desasfaltado al crudo San Fernando, con el objeto de evaluar opciones tecnológicas para su utilización. Para ello se realizó el diseño y puesta en marcha de un sistema de gasificación de asfaltenos a escala de laboratorio, donde se empleó una metodología experimental, realizando nueve pruebas y tomando como variables independientes la temperatura y el agente gasificante (oxígeno). El syngas obtenido de la gasificación fue analizado por dos técnicas de cromatografía, las cuales reportaron la presencia de gases de refinería y azufrados. De estos resultados experimentales se evidencia una tendencia de crecimiento en la composición del CO, H2 y azufrados, y una disminución en la composición del CH4 y CO2 con la temperatura. Se evaluó la composición del syngas con la variación del agente gasificante (33%, 40% y 47% del oxígeno teórico requerido en una combustión total) en cada uno de los niveles de temperatura operados y se estableció que al utilizar un 40% de agente gasificante, se obtienen en promedio los mejores resultados en las composiciones del CO y H4, siendo estos los gases de interés en el proceso de gasificación.
Palabras Clave: crudo San Fernando, gasificación, asfaltenos, syngas.
RESUMEN
Neste trabalho desenvolveuse o primeiro estudo no país da obtenção e avaliação das composições do syngas a partir da gaseificação de asfaltenos provenientes da aplicação do processo de Desasfaltado ao cru São Fernando, com o objeto de avaliar opções tecnológicas para a sua utilização. Para tal realizou-se o desenho e posta em marcha de um sistema de gaseificação de asfaltenos a escala de laboratório, onde se empregou uma metodologia experimental, realizando nove provas e tomando como variáveis independentes a temperatura e o agente gaseificante (oxigênio). O syngas obtido da gaseificação foi analisado por duas técnicas de cromatografia, as quais reportaram a presença de gases de refinaria e enxofrados. Destes resultados experimentais evidenciase uma tendência de crescimento na composição do CO, H2 e enxofrados, e uma diminuição na composição do CH4 e CO2 com a temperatura. Avaliouse a composição do syngas com a variação do agente gaseificante (33%, 40% e 47% do oxigênio teórico requerido em uma combustão total) em cada um dos níveis de temperatura operados e estabeleceuse que ao utilizar um 40% de agente gaseificante, obtém-se em média os melhores resultados nas composições do CO e H4, sendo estes os gases de interesse no processo de gaseificação.
Palavras Chave: cru São Fernando, gaseificação, asfaltenos, syngas.
INTRODUCTION
The decrease of conventional crude oil reserves in Colombia has brought about an incremental use of heavy crude oil reserves (Rubiales, Castilla & San Fernando). Due to their high viscosity, sulfur and metal content, these crude oils represent a challenge for their transportation and refining. Therefore, several technological alternatives are evaluated to utilize them in a very efficient manner. The deasphalting process is one of these alternatives to separate the light and the heavy fractions of crude oil, resulting in improved crude oil with less viscosity, sulfur and metal content as well as a byproduct called asphaltenes (Wallace, Jonson & Thacker, 2001). These asphaltenes have different utilization schemes, being one of them their submission to gasification processes.
Asphaltenes have a molecular weight between 1000 and 2000, their molecule size ranges between 60-90 Armstrong and, in general terms, exhibit a boiling point greater than 540°C (Centeno, Trejo & Ancheyta, 2004; Delgado, 2006). Different studies conclude that asphaltenes are composed of condensed aromatic cores with naphthenic and alkyl substitutions, heteroatoms and metallic compounds that can be dispersed throughout the molecule (Navarro, 2004; Delgado, 2006).
The gasification process has been a topic of research for many years (Liu & Kojima, 2004); its development and commercialization have also been a subject of analysis (Dyk, Keyser & Coertzen, 2006). Today, there are nearly 150 commercial gasification plants in development, construction or operation (Peabody Energy, 2009). However, asphaltenes gasification has not been formally researched, a fact that justifies the undertaking of this study.
The research objective is to complete a design for asphaltenes gasification and the collection and evaluation of the resulting syngas by chromatographic analyses. Based on syngas composition and on the residues generated in the process, syngas mass yield was determined for each trial, thus obtaining yields between 62% and 77%.
THEORETICAL FRAMEWORK
The gasification process consists in submitting feedstocks to high temperatures (800°C -1.800°C) and pressure (Huang, Fang, Chen & Wang, 2003; Higman & Burgt, 2007; Texaco, 1995) with a gasification agent, usually, water vapor, oxygen, air or a mixture of them. The syngas heating value varies according to these components. This feedstock is converted in crude gas (CO2, CH4, H2, CO among others) that is cooled and purified to obtain a mixture of CO and H2 (Leibold, Hornung & Seifert, 2008; Higman & Burgt, 2007; Steynberg & Dry, 2004; Texaco, 1995). Reaction rates in this range of temperature are sufficiently high to be described by the thermodynamic equilibrium modeling of the main gas components and coal, giving results closer to reality. This is the foundation for the design of commercial reactors (Higman & Burgt, 2007).
The asphaltenes gasification process in this paper and in publications from Texaco (Wallace, Jonson & Thacker, 2001), Shell and other companies, is similar to different gasification processes for coal, petroleum residues and biomass (Higman & Burgt, 2007). The asphaltenes gasification process, as other petroleum residues, requires temperatures above 1000°C to obtain the best results (Wallace, Jonson & Thacker, 2001; Wallace, Anderson, Rodarte & Preston, 1998; Deschamps, Dezael & Franckowiak, 1986).
The composition of syngas resulting from asphaltenes gasification has not been reported extensively in any publication. Asphaltenes gasification studies result from deasphalting processes. Therefore, petroleum companies have proposed the integration of deasphalting processes with asphaltenes gasification (Wallace, Jonson & Thacker, 2001; Wallace, Anderson, Rodarte & Preston,1998), thus creating very attractive alternatives for crude improvement and for the application of the byproducts resulting from the deasphalting process (asphaltene). Asphaltene, in turn, is a low-cost raw material that can be submitted to a gasification process in order to increase the profitability of these processes (Wallace, Anderson, Rodarte & Preston,1998).
The main reactions taking place in gasification are: (Marano, 2003; Higman & Burgt, 2007; Ouyang, Guo, Duan, Song & Yu, 2005):
Exothermal Reactions
Partial Combustion
Total Combustion
Water shift
Methanation Reaction
Endothermal Reactions
Boudouard Reaction
Water-gas Reaction
Equations 3 through 6 are reversible. Their equilibrium can be described with the Van’t Hoff Equation that considers the temperature effect over the equilibrium constant and, therefore, over the equilibrium conversion. (Smith, 1991; Smith, Van Ness & Abbott, 1997).
Van’t Hoff Equation (Smith, 1991).
The gasification process is a controlled combustion with a reduced O2 atmosphere, using from one third to one fifth of the O2 required in total combustion (Steynberg & Dry, 2004). Most of the O2 supplied in the gasifier is consumed in the combustion reactions. The water shift reaction is used in the adjustment of the H2/CO ratio. The objective is to maximize the CO and H2 content (Furimsky, 1999; Ouyang et al., 2005; Sharma, Takanohashi, Morishita, Takarada & Saito, 2008; Texaco, 1995). The CH4 can be formed at low gasification temperatures and can be reformed to increase the CO and H2 in the syngas from the reforming reaction (Equation 8) (Marano, 2003; Higman, & Burgt, 2007).
Reforming reaction
Sulfur in the feedstock is mainly converted to small quantities of H2S and COS. Most of nitrogen is converted into N2, with small amounts of HCN and NH3 (Secades, 2003; Hoffman, 2003; Higman & Burgt, 2007; Leibold et al., 2008; Texaco, 1995).
Using deductions of the thermodynamic model, Higman and Burgt (2007) determined the change in gas composition at different pressure values (from 10 bar to 100 bar), keeping a constant temperature of 1000°C. The results of such study indicate that CH4 and CO2 composition in syngas increased with pressure, while CO and H2 production decreased.
Higman and Burgt (2007) repeated the calculation keeping a constant temperature of 1500°C. They observed the same trend as pressure increases, although their conclusion indicates that syngas changes at this temperature are negligible.
In order to determine syngas variation with temperature, (from 1000°C to 1500°C), Higman and Burgt (2007) kept the pressure constant at 30 bar. They observed that, as temperature increases, H2 and CO content also increases and the content of CO2, CH4 and H2O decreases. This conclusion was validated in the asphaltenes gasification.
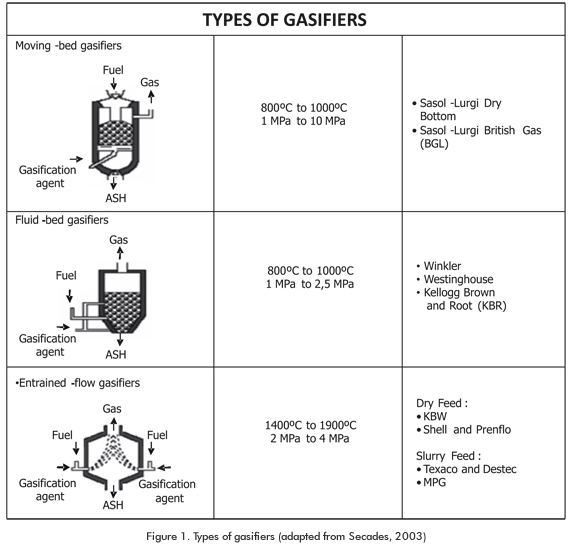
Figure 1 illustrates the available gasification technologies, together with their operation conditions (Dyk et al., 2006; Secades, 2003; Higman & Burgt, 2007; Steynberg & Dry, 2004).
Most of the operating plants were constructed for the production of chemical compounds and synthetic fuel through the Fischer-Tropsch synthesis and the clean production of electric energy (Integrated Gasification Combined Cycle - IGCC) (Marano, 2003; Huang, et al., 2003; Leibold et al., 2008; Steynberg & Dry, 2004).
EXPERIMENTAL DEVELOPMENT
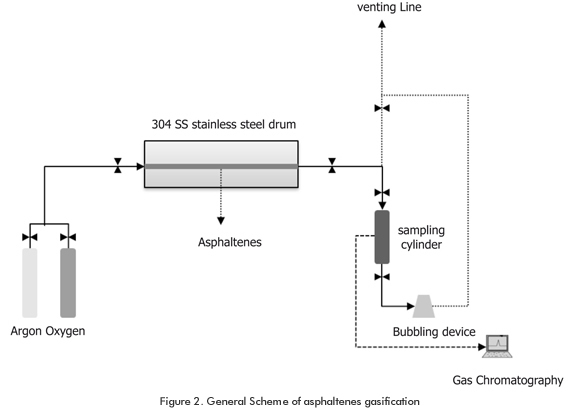
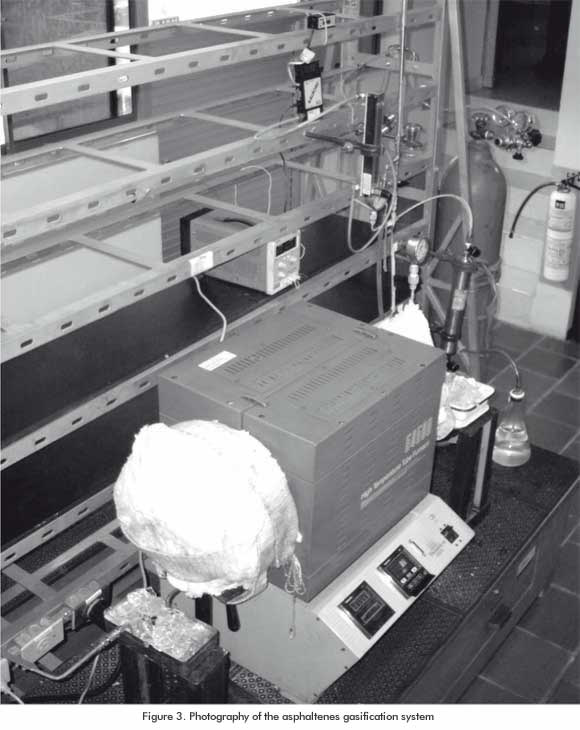
A laboratory-scale asphaltenes gasification system was designed and implemented, consisting basically in a tubular oven (Figure 2). A temperature sensor was located in the electric oven, where the tubular reactor sits. This oven can operate at a maximum of 1500°C and the main characteristics of the reactor are: 304 SS stainless steel drum, with a 6,35 cm external diameter and schedule 40.
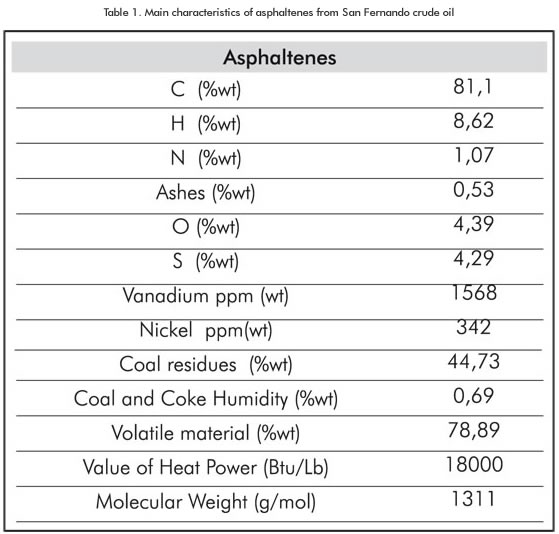
Three basic raw materials were used in the trials: asphaltenes, 99,99% pure oxygen and 99,99% pure argon. The asphaltenes used comes from the San Fernando crude deasphalting process which is classified as an extra-heavy crude oil (8,7 °API). The process was conducted by Instituto Colombiano del Petróleo (ICP) -Ecopetrol S.A. and the main asphaltenes characteristics are listed in Table 1.
The process variables considered were determined based on the BIS BS 4445 regulation and deductions a-ccording to Higman and Burgt (2007). The most influential variables in the process were temperature and amount of gasification agent. A 32 factor design was used (Table 2).
Methodology developed for each trial
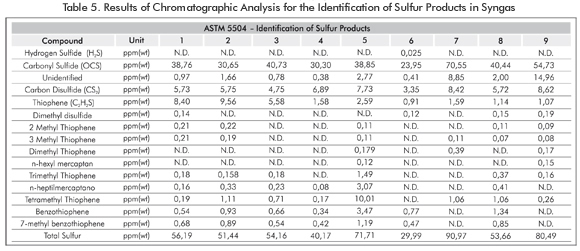
An asphaltenes sample weighing 15g was placed inside the tubular reactor. The inert atmosphere was attained by injecting Argon into the system at a volumetric flow velocity of 500 cm³/min. Then, the reactor input and output valves were closed. Once the system was ready (Figure 3), the heating ramps were programmed. When the sensor reported a temperature of 600°C, the input valve was opened to let the gasification agent (oxygen) enter (Table 2) at 170 psi. The gasification agent was allowed to react with the asphaltenes to the set point temperature (Table 3). This process was conducted in a discontinuous system and the Syngas collection conformed to the Regulation GPA 2166. Once the syngas was collected, chromatographic analysis were conducted at the ICP - ECOPETROL S.A Chromatography Laboratory for the identification of sulfur materials and refinery gas, following the ASTM 5504 Regulation and the UPO 539 Regulation respectively.
The residues left by asphaltenes gasification were collected one day after the trial was done, since it became necessary to wait for the cooling of the reactor.
RESULTS
The results obtained in the asphaltenes gasification trials are shown in Tables 4-6.
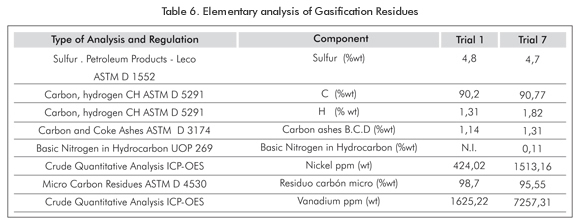
Residues from trials 1 and 7 were analyzed at the Heavy Crude Oil and Spectroscopy Laboratories of (ICP)-Ecopetrol S.A in order to determine their elementary composition.
These trials were selected since they correspond to the highest and lowest levels of temperature with 40% of gasification agent, that is, the best results were reported in these trials.
RESULT ANALYSIS
Carbon conversion was determined for Trials 1 and 7 (Tables 4 and 5).
As explained below, increasing temperature during asphaltenes gasification makes this process more efficient, since it decreases the amount of tar and gasification byproducts; therefore improving syngas composition and carbon conversion. Trial 7 showed higher carbon conversions than trial 1 due to the fact that the residue in trial 7 was 3,66 g while trial 1 was 6 g. (Tables 4, 6, and 7).
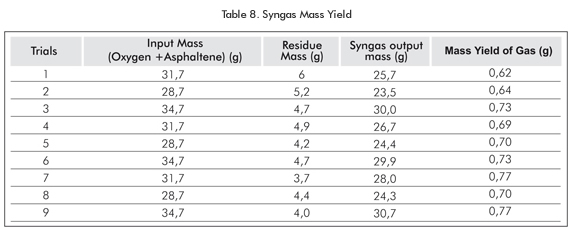
The greatest mass yield of syngas was observed in trials 7 and 9 with 77% each. The lowest mass yield was 62% in Trial 1 (Table 8).
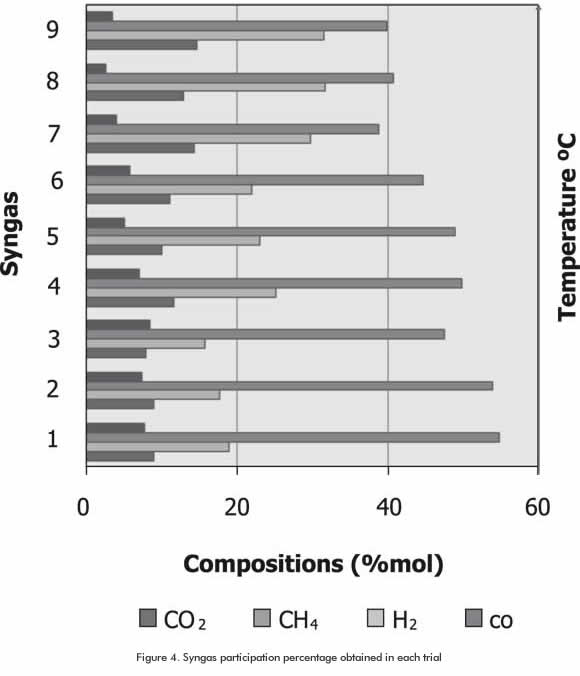
The increasing tendency of CO and H2 proportion remained in each temperature level (Figure 4). Variations in syngas composition with temperature show that CH2 proportion is significant (Table 4). However, as temperature increases, CH4 and CO2 proportion decreases. CO and H2 compositions show increments greater than 57% from the lowest to the highest level of temperature (Figure 5).
It can be concluded that these changes are the result of the effect of temperature on equilibrium conversion (Equation 7), since it was also noted that the equilibrium constant decreased with the increment of temperature in Equation 4 (Methanation reaction). On the other hand, in Equations 5 and 6 (Boudouard and water gas Reactions), an increase of temperature helped the equilibrium constant and, therefore, the equilibrium conversion.
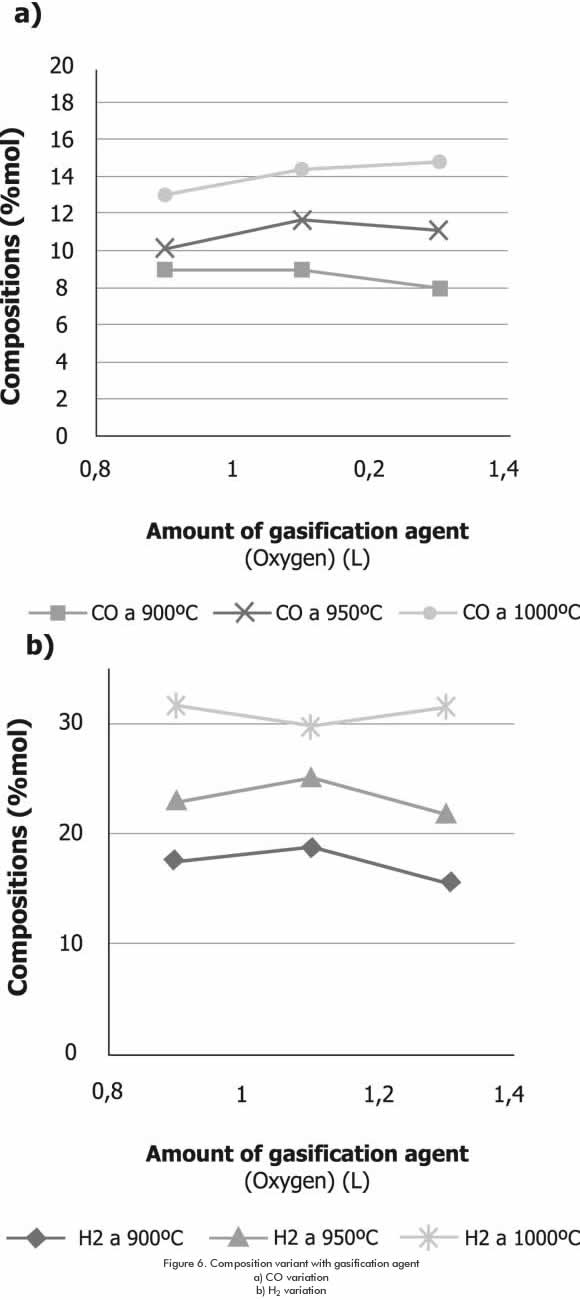
The highest H2 content at temperatures of 900°C and 950°C is attained with 40% of gasification agent, while at 1000°C the highest content is attained with 33% of gasification agent. Regarding CO, at gasification temperatures of 900°C and 950°C, the highest content is attained with 40% of gasification agent, while at 1000°C, the highest CO content resulted with 47% of gasification agent (Figure 6).
It is considered that these changes are generated due to the fact that as temperature increases; the combustion process improves, thus changing the reaction equilibrium.
The greatest H2/CO ratio for the two highest temperatures is obtained with 33% of gasifying agent (Table 4).
The content of sulfur compounds increases as temperature increases due to the breakdown of the asphaltenes heaviest chains which showed the content of sulfur compounds in the feedstock (Table 1). Therefore, the more material is gasified, the less gasification residues are obtained (Table 5) as temperature increases.
The oxygen composition indicates that the heaviest asphaltenes chains did not breakdown completely during the gasification process. Some of them were converted in coke while some of the oxygen supplied remained without reacting. The oxygen reaction increased as temperature increased, due to the fact that more material was gasified (Table 4).
The presence of tars in the process decreased with temperature, being nil at 1000°C. The amount of residues, byproducts of the gasification process, decreases as temperature increases, since the higher the temperature, the more material is gasified. The analysis of residues showed that a portion of them did not contain ash nor carbon and hydrogen (Table 6). This indicates that asphaltenes require higher temperatures to reach complete gasification.
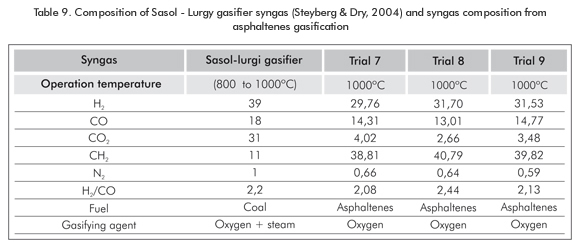
Comparing the results of syngas to the results of Sasol-supplied syngas (Table 9), the H2 and CO compositions are similar to the ones obtained in trials at 1000°C, while CO2 and CH4 amounts greatly vary.
In order to obtain better results in asphaltenes gasification, the use of a mixture of oxygen and water vapor as gasification agent can be implemented, keeping the heat balance of combustion and gasification reactions in the gasifiers, as the gasification agents do (Shell, Texaco, Sasol) (Furimsky, 1999; Marano, 2003; Higman & Burgt, 2007).
CONCLUSIONS
- Results from the asphaltenes gasification process indicate that the greater the temperature, the higher the proportion of H2 and CO and the lower the content of tars and residues. Syngas mass yields reach 77% since there is a greater breakdown of asphaltenes chains, thus improving the gasification process.
- The molar percentage of CO showed a tendency to growth with the increment of the gasification agent at 1000°C, while H2 showed greater molar percentage when 33% and 47% of gasification agent was used at 1000°C. Based on the H2 and CO increase, with the increment of temperature and gasification agent, the best results were obtained when 40% of gasification agent was used.
- The methane composition obtained in the trials was very high if compared to the compositions reported by some gasification plants; although as temperature increased, their proportion decreased from 54% at 900°C to 38% at 1000°C. This proportion is due to the fact that the methanation reaction is exothermal. The amount of CH4 can be decreased by using a mixture of oxygen and water vapor as gasification agent, in order to increase the CO and H2 amount present in the syngas.
- In order to obtain a carbon conversion higher than 75%, the operation temperature must be above 1000°C. Therefore, it is convenient that new stu-dies consider an operation temperature range from 1200°C to 1500°C.
- The reported results for H2 and CO are close to the ones accounted by Sasol. Therefore, the feasibility analysis study for the asphaltene gasification process is considered satisfactory. It is worth mentioning that improvements can be obtained in the results by increasing the operation temperature and using a moderator.
ACKNOWLEDGMENTS
The authors express their gratitude to the Colombian Petroleum Institute (Instituto Colombiano del Petróleo - ICP) -Ecopetrol S.A and Universidad Industrial de Santander, for all the support provided for the development of this research. Furthermore, the authors acknowledge the cooperation given by engineers Jaqueline Saavedra Rueda, Rigoberto Barrero and Maribel Castañeda.
REFERENCES
ASTM D 1552. (2008). Standard test method for sulfur in petroleum products (High-temperature method). [ Links ]
ASTM D 3174. (2004). Standard test method for ash in the analysis sample of coal and coke from coal. [ Links ]
ASTM D 4530. (2007). Standard test method for determination of carbon residue (micro method). [ Links ]
ASTM D 5291. (2002). Standard test methods for instrumental determination of carbon, hydrogen, and nitrogen in petroleum products and lubricants. [ Links ]
ASTM 5504. (2008). Standard test method for determination of sulfur compounds in natural gas and gaseous fuel by gas chromatography and chemiluminescence. [ Links ]
BSI BS 4445. (1969). Schedule of test for gasification and reforming plants using hydrocarbon feedstocks. [ Links ]
Centeno, G., Trejo, F. & Ancheyta, J. (2004). Precipitación de Asphaltene del crudo Maya en un sistema a presión. Sociedad Química Mexicana, (48), 179-188. [ Links ]
Delgado, J. (2006). Asfaltenos.Composición, agregación, precipitación. Mérida ,Venezuela: Escuela de Ingeniería Química, Universidad de los Andes. [ Links ]
Deschamps, A., Dezael C. & Franckowiak, S. (1986). Process for converting heavy petroleum residues to hydrogen and gaseous distillable hydrocarbons. US, 4609456. [ Links ]
Dyk, J.C., Keyser, M. & Coertzen, M. (2006). Syngas production from South African coal sources using sasol-lurgi gasifiers. International Journal of Coal Geology, (65), 243- 253. [ Links ]
Furimsky, E. (1999). Gasification in petroleum refinery of 21st century. Oil & Gas Science and Technology, (44), 567 - 618. [ Links ]
GPA 2166. (1986). Obtaining natural gas samples for analysis by gas chromatography. [ Links ]
Higman, C. & Burgt, M. (2007). Gasification. U.S: Elsevier, 391pp. [ Links ]
Hoffman, Z. (2003). Simulation and economic evaluation of coal gasification with sets reforming process for power production. Msc. Thesis, Louisiana State University, Louisiana, 92pp. [ Links ]
Huang, J., Fang, Y., Chen, H. & Wang, Y. (2003). Coal gasification characteristic in a pressurized fluidized bed. Energy & Fuels, (17), 1474-1479. [ Links ]
Kabe, T., Ishihara, A., Qian, E.V., Sutrisna, I. P. & Kabe, Y. (2004). Coal and coal - related compounds. Structures, reactivity and catalytic reactions. Tokio: Elsevier. [ Links ]
Leibold, H., Hornung, A. & Seifert, H. (2008). HTHP syngas cleaning concept of two stage biomass gasification for FT synthesis. Powder Technology, (180), 265 - 270. [ Links ]
Liu, H. & Kojima, T. (2004). Theoretical study of coal gasification in a 50 ton/day HYCOL entrained flow gasifier. II. Effects of operating conditions and comparison with pilot-scale experiments. Energy & Fuels, (18), 913-917. [ Links ]
Marano, J. (2003). Refinery technology profiles gasification and technologies. Energy Information Administration, U.S. Department of Energy, National Energy Technology Laboratory. [ Links ]
Marano, J. (2003). Refinery technology profiles gasification and technologies. Energy Information Administration, U.S. Department of Energy, National Energy Technology Laboratory. [ Links ]
Navarro, L. (2004). Obtención, caracterización y evaluación de las resinas presentes en el crudo castilla. Tesis de maestría. Universidad industrial de Santander, Bucaramanga, 134pp. [ Links ]
Ouyang, Z., Guo, Z., Duan, D., Song, X. & Yu, X. (2005). Experimental study of coal gasification coupling with natural gas autothermal reforming for synthesis gas production. Ind. Eng. Chem. (44), 279-284. [ Links ]
Peabody Energy (NYSE:BTU) . (2009). Gasification. US. Peabody Energy website. [ Links ]
Secades, A. (2003). Eliminación de H2S de gases de gasificación en reactores de lecho móvil a presión. Tesis doctoral, Universidad de Zaragoza, Zaragoza, 379pp. [ Links ]
Sharma, A., Takanohashi, T., Morishita, K., Takarada, T. & Saito, I. (2008). Low temperature catalytic steam gasification of HyperCoal to produce H2 and synthesis gas. Fuel, (87), 491-497. [ Links ]
Smith, J. M. (1991). Ingeniería de la cinética química. (Sexta edición). México: McGraw-Hill, 774pp. [ Links ]
Smith, J. M., Van Ness, H. C. & Abbott, M. M. (1997). Introducción a la termodinámica en ingeniería química. (Quinta edición). México: McGraw-Hill, 856pp. [ Links ]
Steynberg, A. & Dry. (2004). Fischer-Tropsch technology. South Africa : Elsevier, 700pp. [ Links ]
Texaco. (1995). Texaco gasification process innovative technology evaluation report. U.S. Environmental Protection Agency. [ Links ]
UOP 269. (1990). Nitrogen bases in hydrocarbons by potentiometric titration. [ Links ]
UOP 539. (1997). Refinery gas analysis by gas chromatography. [ Links ]
Wallace, P., Anderson, M., Rodarte, A. & Preston, W. (1998). Heavy Oil Upgrading by the separation and gasification of asphaltenes.Gasification Technologies Conference, San Francisco, California. [ Links ]
Wallace, P., Jonson, K. & Thacker, P. (2001). Integration of solvent deasphalting and gasification. US, 6241874, 05/06/2001. [ Links ]