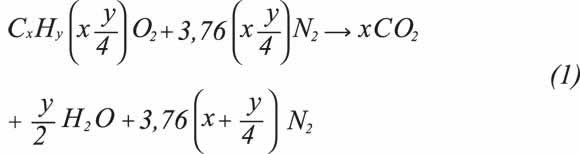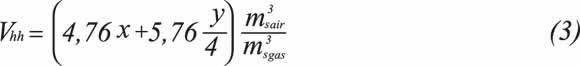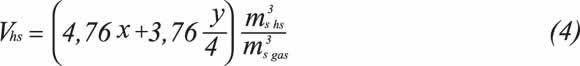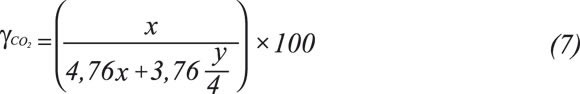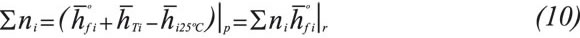Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383On-line version ISSN 2382-4581
C.T.F Cienc. Tecnol. Futuro vol.3 no.5 Bucaramanga Jan./Dec. 2009
COMPARISON OF COMBUSTION PROPERTIES OF SIMULATED BIOGAS AND METHANE
Carlos Díaz-González1*, Andrés-Amell Arrieta2 and José-Luis Suárez3
1 Universidad de Antioquia, Faculty of Engineering. Medellín, Colombia . Member of the Gas Science and Technology and Rational Use of Energy Team (GASURE)
2 Faculty of Engineering. Universidad de Antioquia, Medellín, Colombia . Coordinator of the Gas Science and Technology and Rational Use of Energy Team (GASURE)
3 Faculty of Engineering. Universidad de Antioquia, Medellín, Colombia . Member of the Gas Science and Technology and Rational Use of Energy Team (GASURE)
e-mail: carlosdiaz_13@yahoo.com
(Received March 19, 2008; Accepted October 13, 2009)
* To whom correpondence may be addressed
ABSTRACT
The utilization of new renewable energy sources has been of special interest during the past years, seeking to decrease our dependence on fossil fuels and the corresponding environmental impact derived from their use.
The combustion properties of a simulated gas composed of 60% methane and 40% carbon dioxide in volume are determined in this paper by means of calculation algorithms developed by the GASURE team, comparing them to pure methane properties. Furthermore, the effect of these properties on premixed flame characteristic phenomena is demonstrated. These properties were determined by theoretical estimations. The characteristic phenomena (laminar deflagration velocity, flame structure, radiation pattern) are determined experimentally. Results show a high effect of carbon dioxide in the combustion properties and characteristic parameters of a biogas premixed flame such as laminar deflagration velocity, flame structure and gas-methane exchangeability problems. The difference regarding flame structure and combustion properties lead to a difference in radiation pattern of the gases studied.
Keywords: biogas, combustion properties, premixed flame.
RESUMEN
La utilización de nuevas fuentes de energía renovables ha tenido especial interés en los últimos años buscando disminuir la dependencia de los combustibles fósiles y el impacto ambiental que ellos generan. En este trabajo se determinan las propiedades de combustión de un biogas simulado, compuesto por metano en un 60% y dióxido de carbono en un 40% en volumen, por medio de algoritmos de cálculo desarrollados por el Grupo GASURE y se comparan con las del metano puro. Igualmente se muestra el efecto de estas propiedades en los fenómenos característicos de una llama de premezcla. La determinación de estas propiedades se realizaron mediante estimaciones teóricas y los fenómenos característicos (velocidad de deflagración laminar, estructura de llama, patrón de radiación) se determinaron experimentalmente. Los resultados muestran un gran efecto del dióxido de carbono en las propiedades de combustión y los parámetros característicos de una llama de premezcla de biogas como la velocidad de deflagración laminar, la estructura de llama y problemas de intercambiabilidad entre este gas y el metano. La diferencia en la estructura de llama y las propiedades de combustión conllevan a una diferencia en el patrón de radiación de los gases estudiados.
Palabras Clave: biogas, propiedades de combustión, llamas de premezcla.
RESUMEN
utilização de novas fontes de energia renováveis têm tido especial interesse nos últimos anos buscando diminuir a dependência dos combustíveis fósseis e o impacto ambiental que eles geram. Neste trabalho determinamse as propriedades de combustão de um biogás simulado, composto por metano em um 60% e dióxido de carbono em um 40% em volume, por meio de algoritmos de cálculo desenvolvidos pelo Grupo GASURE comparamse com as do metano puro. Igualmente se mostra o efeito destas propriedades nos fenômenos característicos de uma chama de prémistura. A determinação destas propriedades foi realizada mediante estimações teóricas e os fenômenos característicos (velocidade de deflagração laminar, estrutura de chama, padrão de radiação) determinaramse experimentalmente. Os resultados mostram um grande efeito do dióxido de carbono nas propriedades de combustão e os parâmetros característicos de uma chama de prémistura de biogás como a velocidade de deflagração laminar, a estrutura de chama e problemas de intercambiabilidade entre este gás e o metano. A diferença na estrutura de chama e as propriedades de combustão levam a uma diferença no padrão de radiação dos gases estudados.
Palavras Chave: biogás, propriedades de combustão, chamas de pré-mistura.
INTRODUCTION
Fuel types have experienced a transition process since the beginning of the industrial era, from solid fuels, liquid fuels, to the modern era where the objective is to disseminate the utilization of gas fuels. The objective is to find a renewable, cleaner source of energy, easy to manage and with low environmental impact not only at a local level but also globally. Biogas is one of these renewable sources of energy.
Biogas is produced from the anaerobic fermentation of organic matter in sanitary landfills and in anaerobic biodigestors of organic solid wastes, from plants and animals. The environmental benefit of the production and use of this fuel is deeply appreciated through the reduction of gas emissions that exhibit a greenhouse effect since it is produced by the organic matter decomposition in agricultural and animal wastes as well as in sanitary landfills.
Regarding its use, biogas can be utlized directly as a fuel in heating or power generation processes, as it is stated by Desideri, Di Maria, Leonardo & Proietti (2003) and Zamorano, Pérez, Aguilar & Ridao (2007). The first authors studied the energy potential of biogas produced at a sanitary landfill located in the center of Italy that serves a 400 000 - population town. This work concludes that 60% of the biogas produced at the sanitary landfill can be utilized in internal combustion engines for power generation due to its lower calorific effect, close to 19 000 KJ / Nm3 and given its methane content (greater than 40%). The utilization of the produced biogas represents, according to this publication, a yield close to 100 GW-h/ year. Zamorano et al (2007) conducts a similar work in the city of Granada in the South of Spain, whose landfill serves 300 000 people. An approximate energy potential estimation of 4 500 GW-h/year, greater than the values found in Italy, is reported in this work. This difference is produced because the biogas analysis revealed a 45% methane content in all the biogas produced in the landfill. These works indicate that it is feasible, from the economic standpoint, to utilize the biogas produced in landfills according to the type of technology, being mainly utilized in internal reciprocating combustion engines.
The biogas utilization potential in this type of engines reveals that purification is necessary before considering its use as vehicle fuel (The Society of Motor Manufactu-rers and Traders Ltd. - SMMT - 2002). This purification consists in the elimination of certain components such as hydrogen sulphide, chlorine compounds, and ammonia, among others, and the decrease in the percentage of carbon dioxide. Despite the above, its utilization also depends on the knowledge available about its behavior in combustion systems. Therefore, a phenomenological study of the biogas combustion pattern in premixed systems is required, about its combustion properties and characteristic phenomena in premixed systems, in order to gain enough information to be applied in the design, redesign, or optimization of systems operating with this type of fuel. Furthermore, criteria about optimum atmospheric burner design are necessary so biogas can be utilized efficiently and safely in rural areas where organic materials are available. The objective in this case is to substitute wood logs in the preparation of food, thus contributing to decrease the pressure imposed on rainforest deforestation and avoid respiratory diseases resulting from a deficient wood combustion. Definition of these properties and characteristic phenomena are presented during the development of this work and the methodology described as well.
Combustion Properties and Characteristic Pheno-mena Studied
The estimation of the main combustion properties of gas elements from their chemical composition is convenient to understand the occurrence of a lot of phenomena occurring during combustion processes and to conduct comparative exchangeability analysis. This is achived by applying calculation software, numerical simulation or experimental methods.
The combustion properties of a gas fuel are classified according to the following criteria (Amell, 2002):
- Properties related to air requirement and the formation of combustion products.
- Properties related to the conditions required for combustion and sustainability.
- Properties related to the energy content of a fuel.
The combustion properties related to the first and third criteria are defined as follows (Amell, 2002):
Stoichiometric air volume Va is defined as the air volume, at reference conditions, necessary for the stoichiometric combustion of one cubic meter of gas also at reference conditions.
Relative density d is defined as the ratio between gas density and air density within the same reference state.
The properties related to the generation of combustion products are described as follows:
Volume of wet products Vh is defined as the total volume of combustion products in cubic meters, generated from the total combustion of one cubic meter of gas.
Volume of dry products Vhs is defined as the volume of dry-base combustion products in cubic meters, gene-rated from the total combustion of one cubic meter of gas, that is, without considering the water produced in the reaction.
Volume of water VH2O is defined as the volume of water in cubic meters generated from the total combustion of one cubic meter of gas.
Volumen of carbon dioxide VCO2 is defined as the volume of carbon dioxide in cubic meters generated from the total combustion of one cubic meter of gas.
Maximum carbon dioxide percentage YCO2 is defined as the ratio between the carbon dioxide volume and the dry smoke volume expressed in percentage. This is a maximum value in stoichiometric reaction.
The properties related to the energy content of fuels are described as follows:
Lower Calorific Power (PCI): It is the amount of energy released during the stoichimetric combustion of a fuel. The water in the combustion products is in the gas state.
Upper Calorific Power (PCS). It is the amount of energy released during the stoichiometric combustion of a fuel. The water in the combustion products is in the liquid state.
The difference between PCS and PCI is the water latent vaporization heat.
Wobbe’s Index W: It is the ratio between either the upper or lower gas calorific power and the square root of its relative density. This value is of special interest when analyzing exchangeability among fuel gases.
Adiabatic flame temperature TLL. This is the maximum temperature attained by combustion products when this process is conducted under stoichiometric and adiabatic conditions. While this temperature is never attained in a real combustion process, this is an important property in the study of thermal phenomena.
In this work, the properties are calculated under standard conditions of pressure and temperature (273 K,1atm).
Determination of Combustion Properties
The process begins by balancing the stoichiometric equation with air for a biogas with known composition (prepared and certified by the AGAFANO Company) and for methane (natural gas from reservoirs at Guajira was taken in this case; its composition is shown in Table 1). We applied the calculation algorithms developed by the GASURE team Combugas and Isogas (Amell, 2001) developed by the Gas Science and Technology and Rational Use of Energy Team (GASURE) of Universidad de Antioquia.
For a CxHy gas hydrocarbon product, its stoichiometric combustion is:
Where:
X is the number of atoms of carbon in the fuel.
Y is the number of atoms of hydrogen in the fuel.
On the basis of Equation 1, combustion properties related to air and combustion product requirements are determined by means of Equations 2 to 7, expressed in volumetric terms and the standard reference state.
Theoretical air volume:
Volume of wet products:
Volume of dry products:
Water volume:
Carbon Dioxide volume:
Maximum carbon dioxide percentage:
The calorific power is estimated from calculating the reaction heat or enthalpy for Equation 1, with reactants and products at the same thermochemical reference state. The model used in this calculation is proposed in the ISO 6976 Regulation.
The relative density for a mixture of ideal gases is calculated with the following expression:
Where:
yi is the molar fraction of each gas in the mixture.
di is the relative density of each gas in the mixture.
The Wobbe’s index is calculated with the Equation 9 using the values corresponding to the upper and lower calorific values and the relative densities found with Equation 8.
Where:
PC is the calorific value of the fuel (lower or upper).
d is the relative density of the fuel.
The adiabatic flame temperature is estimated from a balance of the First Law of Thermodynamics, in an adiabatic, stoichiometric reaction with no work interactions and neglecting the kinetic and potential energy changes. Therefore, Hp=Hr. Since reactants enter in the thermochemical reference state, the following equation is obtained:
Where ni is the number of moles of each component in the combustion products.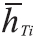 is the enthalpy of each component in the combustion products at the adiabatic flame temperature to be determined and is the enthalpy value at 25°C (298,15K) respectively.
is the enthalpy of each component in the combustion products at the adiabatic flame temperature to be determined and is the enthalpy value at 25°C (298,15K) respectively.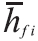 is the formation enthalpy of each component in the combustion products. The adiabatic flame temperature that satisfies the Equation10 for each gas is found by iteration.
is the formation enthalpy of each component in the combustion products. The adiabatic flame temperature that satisfies the Equation10 for each gas is found by iteration.
Determination of Characteristic Parameters of a Biogas Premixed Flame
The characteristic parameters of the biogas premixed flames such as laminar deflagration velocity, flame structure (Amell, 2007) and radiation pattern have been estimated by experimentation, in order to determine the influence of their combustion properties. The methane or natural gas laminar deflagration velocity has been an extensive subject of study (Bradley, Gaskell and Gu, 1996; Illbasa, Crayfordb, Yilmaza, Bowenb, and Syredb, 2006; Gu, Haq, Lawes & Woolley, 2000).
Therefore, no experimentation is conducted on this aspect for such gas, being the measurement of radiation intensity the only experiment conducted with Methane.
Experimental method
The main principles or parameters of premixed flames in laminar regimes are described as follows: laminar deflagration velocity, flame front thickness, blue cone height, flame diffusion height, critical cooling distance, critical cooling diameter, and radiation pattern (Lewis & Elbe, 1987), (Glassman, 1996); (Kuo, 1986); (Turns, 2000); (Baukal, 2000). The following para-meters, among others, have been studied for Biogas and Methane in this paper:
Laminar Deflagration Velocity SL: speed at which the burnt zone combustion advances toward the non-burnt area or velocity at which a fuel releases its energy.
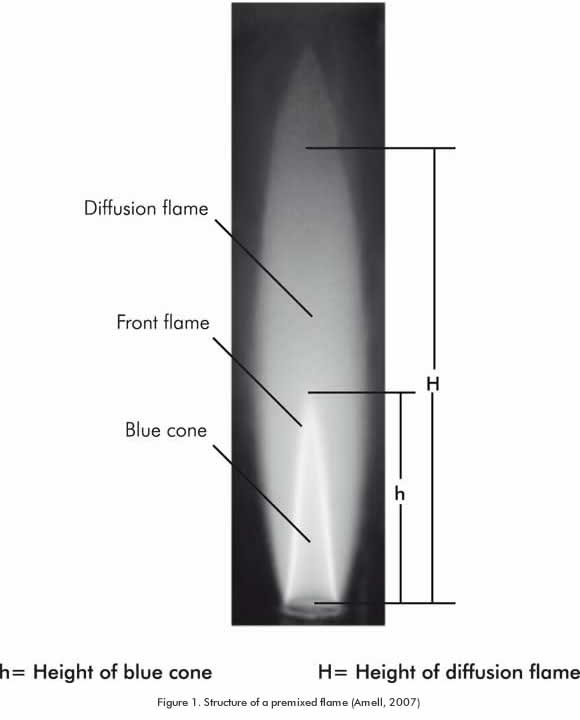
Flame structure: It is composed by a primary combustion zone in the flame front and the secondary combustion zone formed by the chemical reaction of the fuel elements from the flame front and the surrounding oxygen that spreads by diffusion. Figure 1 illustrates the typical structure of a partial premixed flame.
Radiation pattern: This is a non-lighting radiation pattern in non-producing soot hydrocarbon combustion. Its intensity depends on the concentration of CO2 and H2O in the flame volume.
The experimental method followed in this work is similar to the methodology applied in former works conducted and completed by this research team, particularly by Amell, Garcia, Quilindo, and Henao (2004).
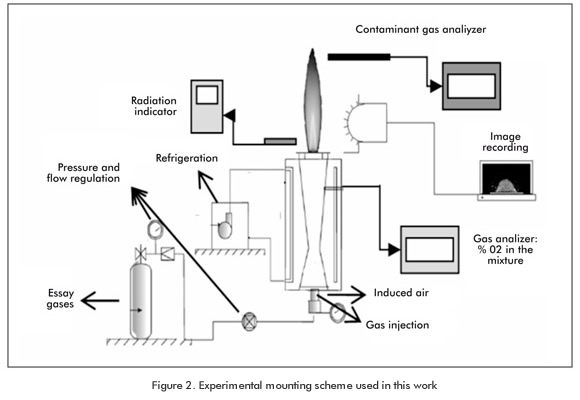
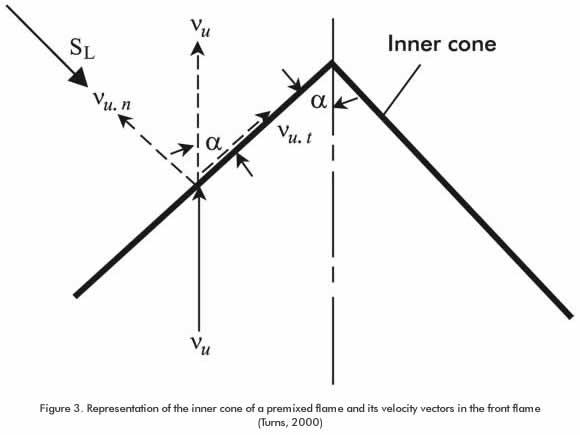
Figure 2 illustrates an experimental mounting scheme utilized in the study of characteristic parameters of a biogas premixed flame. The following equipment was used: an induced - air atmospheric burner, a Landtec gas analyzer for the determination of the composition of the premix, and a wet flow meter. The experiments conducted for estimating the laminar deflagration velocity and the flame structure were conducted by taking images of the flame structure itself, using a high-resolution camera and applying the internal cone angle method shown in Figure 3.
Measurement of flame radiation was accomplished by following the Baukal and Gebhart’s (1997) methodology, using a MedTherm radiometer, taking measurements in axial and radial directions with the burner edge as reference. The blue cone height, the primary ventilation rate and the discharged gas flow were determined in each assay experimentally. Key values such as the stoichiometric air volume and the primary ventilation factor were determined keeping gas composition fixed. The blue cone images were analyzed by using the MATROX INSPECTOR software for image treatment. The flame inner cone height and angle in each assay were determined by this means. The deflagration velocity was determined with these information using:
Where Vu is the velocity of the gas-air mixture and α is angle of the flame inner cone.
The thermal power and the primary ventilation rate were kept constant in the assays measuring flame radiation intensity of both gases, in order to compare values in the same points of burner operation.
Table 2 shows the experiments conducted, the variables to be measured and controlled, and the number of replicas of each experiment.
Results
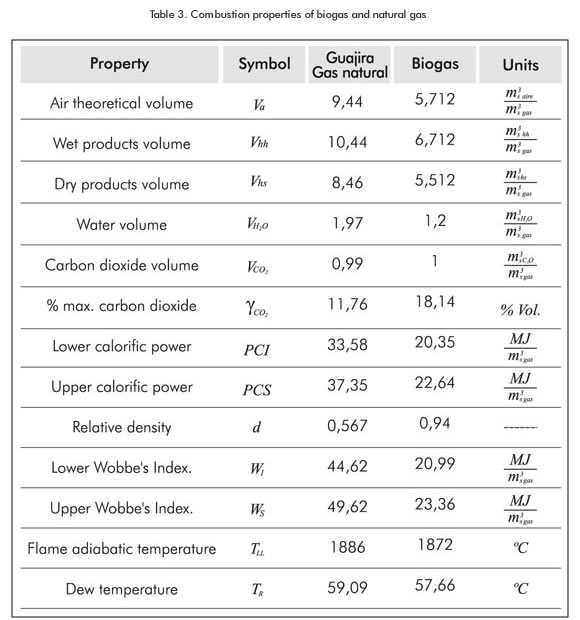
The results pertaining to the determination of the biogas combustion properties and for natural gas are shown and compared in Table 3. These results have been obtained by applying the Combugas and Isogas calculation programs developed by the Gas Science and Technology and Rational Use of Energy Team (GASURE).
The differences in stoichiometric air volumes play an important role in the maximum CO2 percentage, the smoke volume, water volume and in the Wobbe’s index. These properties are lower for biogas; this is explained by the lower proportion of methane in biogas and the incidence of carbon dioxide in the decrease of heat of reaction. However the CO2 incidence in the increase of specific gravity of biogas is because its upper molecular weight of carbon dioxide compared to methane. This also produces that the percentage of CO2 in biogas combustion products is higher than methane's. The significant differences in these properties and in the deflagration velocity value are analyzed below. These differences explain why biogas is not exchangeable with natural gas in combustion equipment.
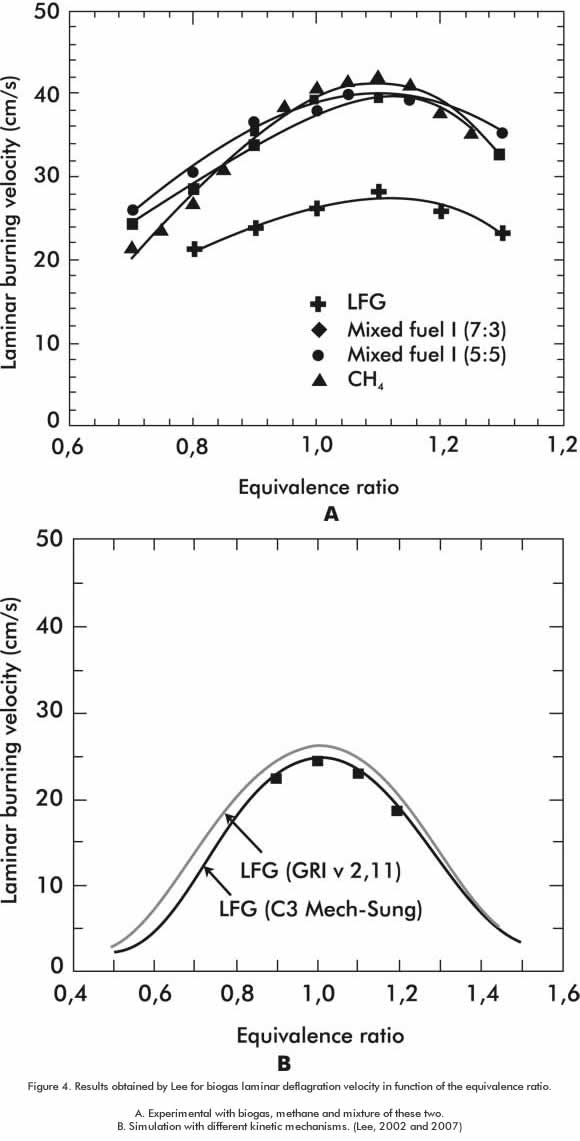
The results obtained in the experimental determination of laminar burning velocity S, agree with other related works shown in Figure 4 (Lee, Oh, Jung & Park, 2002) ( Lee & Hwang, 2007) (Qin, Egolfopoulos & Tsotsis, 2001) and in Figure 5. In this graph, we present the relation of this parameter to the primary ventilation factor n (is the inverse of equivalence ratio). Biogas laminar deflagration velocity graphs are obtained in function of the equivalence ratio that is the inverse value of the ventilation factor n.
An obvious difference between biogas and natural gas, regarding their laminar deflagration velocity behavior, is observed. For instance, the value is approximately 40 cm for natural gas near the stoichiometric mixture and at atmospheric pressure conditions, as it is reported by the publications mentioned above. For biogas, the value is 27 cm. These differences are due to the high level of carbon dioxide in the biogas that, in turn, results in the decrease of laminar deflagration velocity.
In rich mixture conditions, biogas has a greater variation of laminar burning velocity than lower mixtures conditions, behavior that is more symmetrical and less perceptible in methane. The greater concentration of carbon dioxide in rich mixtures conditions, generates lower flame temperatures, which decreases the reaction rate and consequently affects the laminar burning velocity.
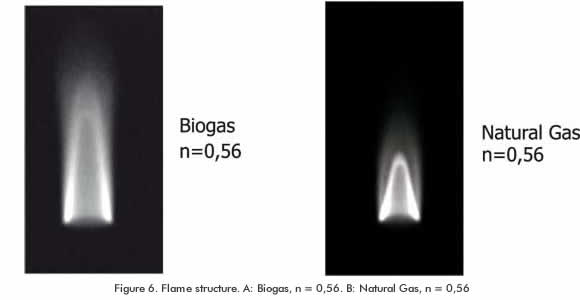
Regarding flame structure, the biogas exhibits notorious differences compared to the natural gas, as it is observed in Figure 6. The height of the inner cone in the biogas primary combustion zone is higher than in natural gas. This is due to the difference in the deflagration velocity mentioned above; the height of the inner cone of a partial premixed flame is inversely proportional to the laminar burning velocity (Amell, 2007). This situation explains why the size of a premixed biogas flame is greater than the biogas flame when the thermal power and the ventilation rate remain constant in the combustion system. An important implication of this result is in the utilization of a combustion chamber designed for methane combustion of biogas. Interferences between the flame and walls can appear, therefore changing the aerodynamics conditions.
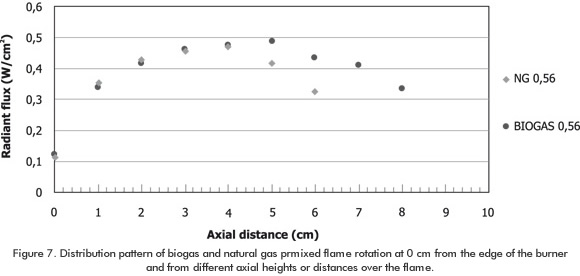
Figure 7 shows the radiation behavior of a biogas premixed flame and a natural gas flame when the thermal power and the primary ventilation rate remain constant. The results show that the biogas has a flame radiation distribution in a broader zone than natural gas, and a greater lighting radiation intensity. This is due to a higher concentration of carbon dioxide and water vapor, since a greater volumetric flow is necessary in biogas, to ensure the same thermal power than natural gas.
According to the flame structure, the maximum emission of radiant heat is produced near the maximum high of the inner cone of the partial premixed flame.
The variation of the radiation intensity is the result of the appearance of species with height emissive power like carbon dioxide and water vapor; in the burner, the concentrations these are low, but with the increase of height of the flame, those concentrations increase until a maximum in the tip of the inner cone. In upper heights, the diffusion of these species decrease its concentration, therefore, the intensity of flame radiation.
CONCLUSIONS
- The results obtained in this research show that the calculation algorithms developed by the GASURE team are helpful in the management of gas fuels and their applications.
- The biogas combustion properties exhibit far different values in comparison with natural gas data, even if both are located within similar magnitudes. The most important differences are less air requirement for combustion, greater percentage of carbon dioxide in combustion products -although the total volume is lower-, decrease in the calorific power and the Wobbe’s index. This is precisely the main factor to be taken into account when analyzing the exchangeability of two gas fuels.
- These differences are revealed in the characteristic patterns of premixed flames where the effect of carbon dioxide in the biogas is evident in the form of a reduction in the laminar deflagration velocity that, in turn, results in changes in flame structure and the radiation pattern observed. The differences observed in flame structure and radiation pattern produced by adding CO2 must be taken into account in the design of household ovens or combustion chambers where biogas is utilized. The results obtained agree with the findings of Lafay, Cabot,and Boukhaifa (2006) about biogas combustion in a gas turbine combustion chamber.
- As a complementary work, the need of studying flame stability is proposed since the lower biogas deflagration velocity value implies less stability than in methane. This parameter determines the behavior of other stability characteristics such as the velocity critical gradients.
- Finally, the results of this work show the importance of knowing biogas behavior as an alternative fuel in premixed systems. The effects of carbon dioxide in this gas show the importance of modifying the combustion systems when a natural gas- biogas exchange is planned.
ACKNOWLEDGMENTS
The authors acknowledge the contribution of the Gas Science and Technology and Rational Use of Energy Team (GASURE) of the Faculty of Engineering at Universidad de Antioquia and the 2009-2010 sustainability program of the Research Vicerectory for its scientific, technological, logistic, and financial support for the completion of this research project ,whose results are partially presented here.
REFERENCES
Amell, A. (2001). CombuGas V 2.0. Calculation software. Gas Science and Technology and Rational Use of Energy Team. Universidad de Antioquia. Facultad de ingeniería. [ Links ]
Amell, A. (2001). IsoGas V 1.0. Calculation software. Gas Science and Technology and Rational Use of Energy Team. Universidad de Antioquia. Facultad de ingeniería. [ Links ]
Amell, A. (2002). Estimación de las propiedades de combustión de combustibles gaseosos. Universidad de Antioquia. Facultad de ingeniería. Centro de Extensión Académica CESET. Medellín. 76 p. [ Links ]
Amell, A., García, J. M., Quilindo, A. & Henao, D. A. (2004) Influencia de la altitud sobre la velocidad de deflagración del gas natural. Revista Facultad de Ingeniería Universidad de Antioquia. 32: 72-81. [ Links ]
Amell, A. (2007). Influence of altitude on the height of blue cone in a premixed flame. Applied Thermal Engineering, 27 (2-3), 408-412. [ Links ]
Baukal, C. (2000). Heat Transfer in Industrial Combustion. USA : CRC Press. 65-108; 195-206. [ Links ]
Baukal, C. & Gebhart, B. (1997). Oxygen-enhanced/natural gas flame radiation.Inf. J. Heat Mass Transfer, 40: 2539-2547 Elsevier Science Inc.. [ Links ]
Bradley, D., Gaskell, P. H. & Gu, X. J. (1996). Burning Velocities, Markstein Lengths, and Flame Quenching for Spherical Methane-Air Flames: A Computational Study. Combustion and Flame, 104: 176-198 Elsevier Science Inc. [ Links ]
Desideri, U., Di Maria, F., Leonardo, D. & Proietti, S. (2003). Sanitary landfill energetic potential analysis: a real case study. Energy Conversion and Management, 44:1969-1981. Elsevier Science Inc. [ Links ]
Glassman, I. (1996). Combustion. (3a ed.) USA : Academic Press. 119-181. [ Links ]
Gu, X. J., Haq, M. Z., Lawes, M. & Woolley, R. (2000). Laminar Burning Velocity and Markstein Lengths of Methane-Air Mixtures. Combustion and Flame, 121:41-58. Elsevier Science Inc. [ Links ]
Ilbasa, M., Crayfordb, A.P., Yilmaza, I., Bowenb, P.J. & Syredb, N. (2006). Laminar-burning velocities of hydrogen-air and hydrogen-methane-air mixtures: An experimental study. Int. J. of Hydrogen Energy, 31: 1768-1779. Elsevier Science Inc. [ Links ]
ISO 6976: (1995). Natural gas - Calculation of calorific values, density, relative density and Wobbe index from composition. [ Links ]
Kuo, H. (1986). Principles of combustion. USA : Wiley-Interscience Publication. 285-332. [ Links ]
Lafay, Y., Cabot, G. & Boukhalfa, A. (2006). Experimental study of biogas combustion in a gas turbine configuration. 13th Int. Symp. On Appl. Laser Techniques to Fluid Mechanics. Lisbon, Portugal . 35.5. [ Links ]
Lee, C. E., Oh, C. B., Jung, I. K. & Park, J. (2002). A study on the determination of burning velocities of LFG and LFG-mixed fuels. Fuel, 81: 1679-1686. Elsevier Science Inc. [ Links ]
Lee, C. E., & Hwang, C. H. (2007). An experimental study on the flame stability of LFG and LFG-mixed fuels. Fuel, 86: 649-655. Elsevier Science Inc. [ Links ]
Lewis, B. & Elbe, G. (1987). Combustion, Flames and Explosions of Gases. (3a ed.) USA : Academic Press. 215-414. [ Links ]
Qin, W., Egolfopoulos, F. N. & Tsotsis, T. T. (2001). Fundamental and environmental aspects of landfill gas utilization for power generation. Chem. Eng. J, 82.: 157-172. Elsevier Science Inc. [ Links ]
The society of motor manufacturers and traders limited SMMT. (2002). Towards a shared vision - Future fuels and sustainable mobility, Londres. 84 p. [ Links ]
Turns, S. (2000). An Introduction to Combustion. (2a ed.) USA : McGraw-Hill. 253-299. [ Links ]
Zamorano, M., Perez, J., Aguilar, I. & Ridao, A. (2007) Study of Energy potential of the Biogas produced by an urban waste landfill in southern Spain. Renewable & Sustainable Energy Reviews, 11(5): 909-922. Elsevier Science Inc. [ Links ]