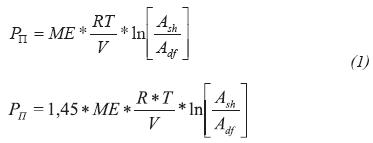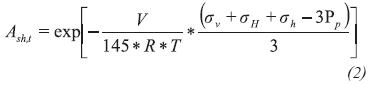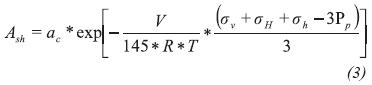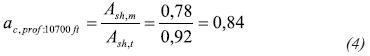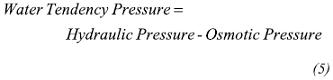Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383On-line version ISSN 2382-4581
C.T.F Cienc. Tecnol. Futuro vol.4 no.1 Bucaramanga Jan./July 2010
1Convenio Ecopetrol S.A. - Universidad Industrial de Santander, UIS, Bucaramanga, Santander, Colombia
2,3Ecopetrol S.A. - Instituto Colombiano del Petróleo, A.A. 4185 Bucaramanga, Santander, Colombia
e-mail: wafv12@hotmail.com reinel.corzo@ecopetrol.com.co
(Received, April 24, 2009; Accepted June 16, 2010)
* To whom correspondence may be addressed
ABSTRACT
Wellbore instability in shales is attributed to many factors. Two of them are mechanical effects and physico-chemical effects. Drilling and drilling fluid cause physico-chemical interaction and the flux of water and ions that may alter the shale stress state through pore pressure and shale strength. This paper presents the analysis of the chemical osmosis phenomenon between drilling fluids and shale formations to evaluate the chemical parameters necessary for modeling the aqueous flux. These parameters are the drilling fluid activity (Adf), shale activity (Ash) and membrane efficiency (ME).
This work also characterizes the shales for drilling purposes and describes an integrated methodology to obtain the magnitude of the chemical parameters. Furthermore, it is stated how the generation of effective osmotic pressure between the formation and drilling fluid define the water flux direction. Finally, the application of the results of the chemical analysis to Carbonera shale is presented. The design of laboratory tests for two mud formulations, Mud A and Mud B, and the field application is also showed. The Mud A is a balanced activity mud and the Mud B is a high salt concentration mud which may produce water flux out of the shale formation (dehydration) during drilling, in some sections of the wellbore, increasing the formation strength. The results presented in this paper will help to reduce the risks associated with wellbore instability during the drilling of shale formations and thereby lowering the overall non-productive time and reducing drilling costs.
Keywords: drilling fluids, osmotic pressure, chemical osmosis, shales, well stability.
RESUMEN
La inestabilidad de pozo en formaciones arcillosas es atribuida a muchos factores. Dos de estos factores son los efectos mecánicos y físico-químicos. La perforación y el fluido de perforación causan una interacción físico-química a través del flujo de agua e iones que pueden alterar la presión de poro, el estado de esfuerzos in-situ y la resistencia de la roca. Este artículo presenta el análisis del fenómeno de ósmosis química entre el fluido de perforación y la formación arcillosa para evaluar los parámetros químicos necesarios para modelar el flujo acuoso. Estos parámetros son la actividad del fluido de perforación (Adf), actividad de la formación arcillosa (Ash) y la eficiencia de membrana (ME).
Este trabajo también caracteriza la formación arcillosa para propósitos de perforación y describe los métodos para obtener la magnitud de los parámetros químicos. Así mismo se establece como la generación de una presión osmótica efectiva entre la formación y el fluido de perforación define la dirección del flujo acuoso. Finalmente se presentan los resultados del análisis químico para la formación Carbonera. Se expone también las pruebas de laboratorio y la aplicación en campo para dos formulaciones de lodo, Lodo 'A' y Lodo 'B'. El lodo 'A' es un lodo de actividad balanceada y el lodo 'B' es un lodo de alta concentración salina el cual puede producir una deshidratación de la formación durante la perforación, en algunas secciones del pozo, aumentando la resistencia de la formación. Los resultados presentados en este artículo muestran como la selección de la adecuada salinidad de la fase acuosa en un fluido base aceite (OBM) ayudará a reducir el riesgo asociado a inestabilidad durante la perforación de formaciones arcillosas y por lo tanto disminuir los costos de perforación y el tiempo asociado a improductividad de pozo.
Palabras clave: fluidos de perforación, presión osmótica, osmosis química, shales, estabilidad de pozo.
INTRODUCTION
The wellbore instability in shales is of great interest to drilling operations in Colombian foothills due to the fact that they are the most troublesome and abundant formations. The shale instability is strongly influenced by the drilling mud chemical interaction. This interaction can lead to wellbore instability problems caused by the particular properties of shales among which the most relevant ones are the amount and type of mineral composition, negative surface charge, low permeability and highly-laminated structure. Because of the fluidrock interaction, the effective stress distribution nearby the wellbore and the rock strength changes by the water flux (pore pressure variation) and ionic transport.
This paper describes the chemical parameters that quantitatively affect the water flux between the drilling fluid and the shale, establishing new pore pressure around the well. To do this, the osmotic pressure is calculated for the problematic zone in the Carbonera formation in a well located in Colombian foothills, where well-costs and rig-time are associated mainly with wellbore instability problems.
The chemical wellbore instability study optimizes the mud formulations according to different types of shale to establish better drilling conditions. The different scenarios with the formulated Mud A and Mud B demonstrated how the chemical activity in the water phase of oil-based mud can generate a favorable osmotic pressure across the shale. This osmotic pressure gradient affects the water flow tendency, thus alter near wellbore pore pressure and local stress state.
DRILLING FACTORS AFFECTING THE SHALE INSTABILITY
Abbas, Shebatalhamd, Khan, Al-Shobaili, Ansari, Ali & Mehta (2006) and Zhang, Clark, Al-Bazali, Chevenert, Sharma, Rojas & Ong (2006) defined the principal factors that affect the wellbore to become unstable after the borehole is drilled, such as in-situ stress field, well trajectories, rock properties, drilling fluid properties (chemical and mud weight), formation temperature and time spent in open hole. For the drilling engineer, some of these factors are controlled during the drilling, such as mud density, chemical mud composition, well trajectory and drilling practices.
An important tool to help to maintain the wellbore stability is the Mud Weight Window (MWW). The MWW provides mud weight ranges which define whether the behavior of the borehole is stable or not. Ideally a mud weight greater than the pore pressure and the shear failure gradient (to avoid kicks and breakouts) and less than the tensile failure gradient (to avoid fracturing the formation) is chosen. Following the mud weight program based on the MWW (Figure 1) the mud weight between the hole collapse (solid line) and tensile failure (long spaced dashed line) must be maintained. The mud pressure line represents the optimal mud weight to use for drilling. However this MWW is altered due to chemical interactions between the drilling fluid and shale formation. Carminati, Del Gaudio, Zausa & Brignoli (1999), Al-Bazali (2005), Zhang et al. (2006) and Corzo (2009) showed that shale/ mud interaction involves water and ion movement, which changes pore pressure distribution near wellbore and rock strength. The MWW variation is shown in Figure 1 (b and c). Chemical effects caused reduction of the safe mud window as shown in Figure 1b, while an increase of the material cohesion and decrease in the pore pressure caused enlarging the mud weight window, Figure 1c.
Therefore, in shale formation the drilling fluid chemical effects must be considered in addition to the mechanical effect (mud weight). The chemical effects are related to the movement of water/ions into or out of shale. Movements controlled by chemical and hydraulic gradients which will be introduced as follows.
PHENOMENOLOGY OF WATER FLOW
Hydraulic and chemical potential gradient are recognized as the two main mechanisms to control water flow into or out of shale formation. The hydraulic pressure difference between drilling fluids and shale causes bulk flow (diluent and solute) of drilling fluid and is governed by the Darcy Equation; this flow is called hydraulic conduction, convective flow, pore pressure penetration or pore pressure diffusion. During overbalanced drilling, the hydraulic water flow developed tensile failure in the wellbore due to pore pressure increase. One advantage of OBM's over the WBM's is the restriction of water flow due to the existence of a threshold capillary entry pressure between oil-based mud and the water-wet shale.
The chemical osmosis occurs when the chemical potential gradient is the mechanism that causes the movement of water (diluent). The driving force is determined by the water activity difference between drilling mud and shale pore fluid. The water flow tendency is from high water activity (low-salt concentration) to low water activity (high-salt concentration). According to Ghassemi, Diek & Santos (2001) the osmosis transport is often several times greater than the contribution of the hydraulic fluid transport, therefore the chemical osmosis will be quantified to evaluate the optimal mud formulation to reduce the water hydration in these formations.
CHEMICAL OSMOSIS
The osmotic flow describes the water flow developed when solutions of different chemical activity are separated by membrane; in this case, the solutions are the drilling fluid and pore fluid in the shale. The water flow generates osmotic pressure that is quantified by Equation 1.
Where:- Pπ = Effective osmotic pressure, psi
ME = Membrane efficiency, %
T = Temperature, K
V = Molar volume of water, 18 cm3
R = Gas constant, 8,314 Pa m3/mol K
Ash = Water activity of shale formation
Adf = Water activity of drilling fluid
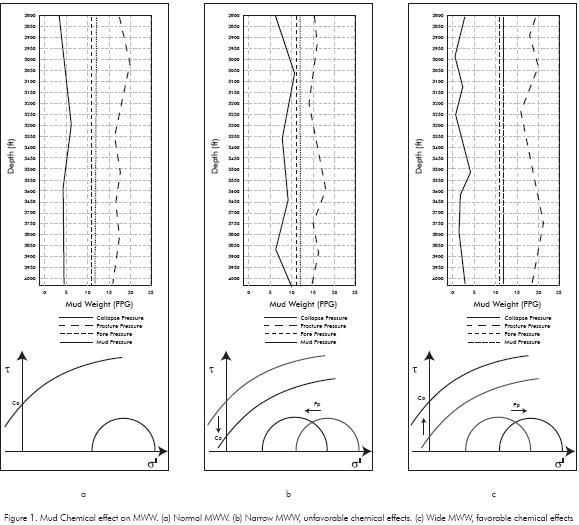
If the osmotic pressure value is positive, shale dehydration occurs. Starting from the water activity differences between drilling fluid and shale formation, it is possible to determine three cases on water flow as shown in Figure 2. These cases are being analyzed under the condition of zero pressure gradients between hydraulic mud pressure and pore pressure. In case 1 of Figure 2 the water activity of the shale formation (Ash) is lower than the water activity of the drilling fluid (Adf), the osmotic pressure is negative and net water tendency is forwarded the shale. Case 1 produces an increase in pore pressure near wellbore, effect that is time-dependent as demonstrated by Ghassemi & Diek (2003) and Oort, Hale, Mody & Roy (1996). The water activity in the drilling fluid is the same as shale formation activity in case 2, where the osmotic flow is zero. This concept is the basis for the balanced activity mud formulation (Chenevert, 1970). In case 3 (Ash > Adf) the osmotic pressure is positive and the water is osmotically extracted (shale dehydration) which could lead to pore pressure decrease and strength shale increase.
In order to built a high and positive effective osmotic pressure the chemical gradient between the drilling fluid and shale formation must be maintained Figure 2. Chemically induced water flow in shale formations by high membrane efficiency. Fritz & Marine (1983), Ballard, Beare & Lawless (1992), Oort, Hale, Mody & Roy (1996), Lomba, Chenevert & Sharma (2000); Tan, Drummond, Mody & Tare (2002), Al-Bazali (2005), Fernández (2008) experimentally showed that shales are not ideal semi-permeable membranes and their value depends on rock and drilling fluid properties.
CARBONERA FORMATION CHARACTERIZATION
The Colombian foothills in South America are an important area for hydrocarbon exploration (Figure 3). The instability problems can be generated when drilling in this complex area characterized by high horizontal stresses, alternate sequences of shales and shaly-sands and tectonically active region. Taking into account the drilling operations problems in this zone which are related to Carbonera shale formation, the chemical analysis was applied to this formation. The first step in the chemical analysis to Carbonera Formation in the Well U-10 is to do a chemical characterization of the formation. The preserved Carbonera shale cored from 10 700 ft of depth from the U-10 well were used in our work.
Mineralogical composition
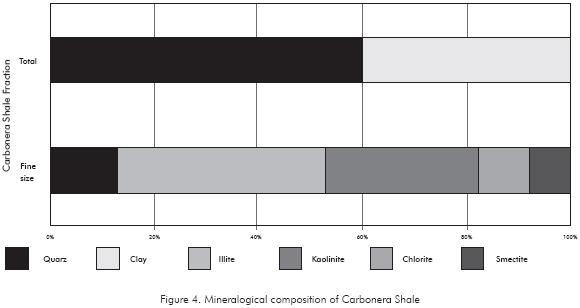
The qualitative mineralogical composition of the Carbonera formation was obtained through the method of X-Ray Diffraction, carried out for both total (less than 2mm) and fine grain size fraction (less than 0,074mm). The Figure 4 shows a summary of the chemical analyses for the Carbonera Core. The rock is generally composed of quartz (60%) and clay (40%); the clay mineral predominant in this shale is Kaolinite, Illite and microcrystalline quartz.
Native moisture content and water activity
The native moisture content was estimated once the preserved U-10 shale is removed out of its wrapping. The method used to obtain the native moisture content was based on the initial weight of one piece of shale and the final weight after the shale sample was dried by placing it inside an oven at 200°F (93°C) for 24 hours. The native moisture content for each shale sample was calculated based on the weight differences.
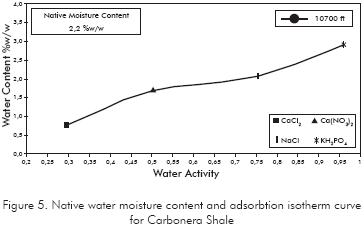
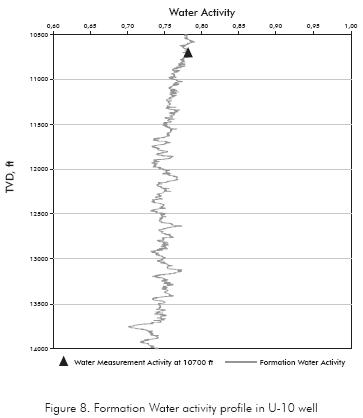
To determine the shale native water activity the adsorption isotherm curve was elaborated for the Carbonera shale. The adsorption isotherm curve is a plot of weight percentage of water adsorbed by shale when it is placed in various desiccators of different activity. The Figure 5 shows the adsorption isotherm curve for the Carbonera formation and the native water content. The native shale water activity is determined by matching the native water content of the shale with its respective water activity value in the adsorption isotherm plot. The water activity obtained for the Carbonera shale was 0,78, value that is similar to the one obtained by Reyes & Vargas (2005) where the water activity for the Carbonera C8 formation was 0,7.
The above method measures the water activity at atmospheric pressure and temperature. However, it was found that the water activity of shales changes with pressure and temperature (Chenevert & Strassner, 1975; Fonseca & Chenevert, 1998; Zhang et al., 2006; Yu, 2002).
CHEMICAL PARAMETERS
The three chemical parameters for the effective osmotic pressure determination are the drilling fluid activity (Adf), pore fluid shale activity (Ash) and membrane efficiency (ME). The chemical force, potential or gradient is defined by the differences between the drilling fluid and shale pore fluid activities. These chemical parameters and the effective osmotic pressure were calculated for the well U-10 interval 10 500-14 000ft.
Water activity of OBM drilling fluid
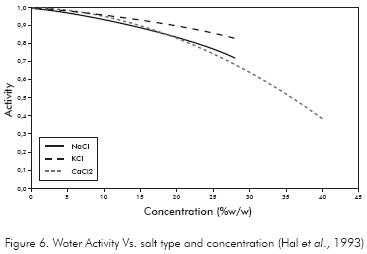
Hale, Mody & Sallsbury (1993) proposed several easy-to-use correlations to calculate the mud activity as a function of the salt type and concentration in the aqueous solution of the drilling fluid. These relations are shown in Figure 6 and must be used only for the drilling fluid activity since the formation water have not only one type of ion as demonstrated by Santarelli, Chenevert & Osisanya (1992); Carminati et al. (1999); Mody, Tare, Tan, Drummond & Wu (2002) and Da Fontoura, Rabe & Lomba (2002), who found anions as Ca+2, Mg+2, Na+, K+ and cations like Cl- y SO4 -2 in the water formation.
The correlations above mentioned estimate the mud activity at 25°C (77°F) and atmospheric pressure.
Water activity of Carbonera Formation
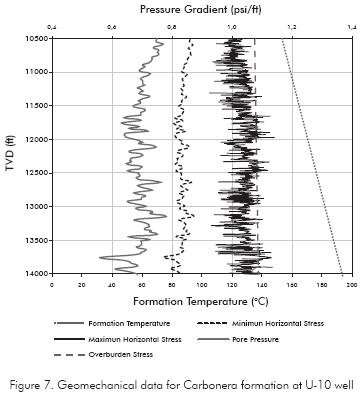
The formation activity is an indicator of hydration state and its capacity to adsorb water. It is important to note that determinating water activity of the shale is difficult to obtain in their in-situ conditions since these values depend on temperature, pressure and the components of pore fluid. To determine the shale formation activity, the modified method of effective mean stress proposed by Zhang, Rojas & Clark (2008) which uses geomechanical data to generate a record of activity with depth, was used.
The geomechanical data of this method consists of in-situ stresses, pore pressure and formation temperature. In Figure 7, we can see a detailed description of it for the drilled section.
Once obtained the geomechanical data, the theoretical formation water activity is calculated using the following equation:
Then the corrected water formation is calculated using the following equation:
Where:
- Ash = Formation water activity
ac = Correction coefficient
sv = Overburden stress, psi
sH = Maximum horizontal stress, psi
sh = Minimum horizontal stress, psi
PP = Pore pressure, psi
The ac (water activity correction coefficient) calibrates the activity values from data obtained from the adsorption isotherm curve. This correction coefficient at 10 700 ft is defined as follows:
Where:
Ash,m = Measured shale water activity from laboratory data
Ash,t = Theoretical activity of the rock obtained from Equation 2
The Figure 8 shows the shale water activity prediction.
Membrane efficiency
The membrane efficiency, reflection coefficient or ionic perm-selectivity describes the property of the shale to restrict ion movement when interacting with drilling fluids; it also describes the membrane capacity to sustain osmotic flow between the drilling fluid and the shale formation. If the shale completely blocks the ionic flow, then the shale is referred to as a perfect ionselective membrane with membrane efficiency equal to 100%. If the shale allows ions freely to move in or out of the shale depending on the direction of the chemical gradient, the shale is a non-selective membrane with efficiency equal to 0% as seen in Figure 9.
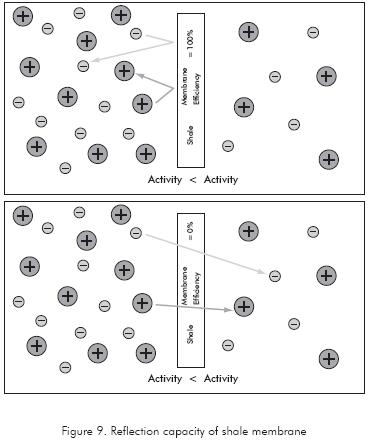
The membrane efficiency depends on both the rock properties and the drilling fluid. Rock properties like CEC, permeability, and pore throat; and fluid properties like ratio of the hydrated ion (solute) define the membrane efficiency values in the rock-drilling fluid system as stated by Zhang et al. (2008).
If the membrane efficiency is low, the ions will tend to move from high concentration to low concentration; this movement tends to reduce the difference in water chemical potential and thus eliminates the osmotic forces. The shale rock formation really acts as leaky semi-permeable cationic membrane; it means that the membrane is selective to cations but not perfect. In other words, the shale has allowed cations and some anions to pass through.
When the shale formation interacts with Water Base Muds the rock behaves as a non-perfect semi-permeable cationic membrane due to characteristics of low permeability in the range of nano-Darcies (Al-Bazali, 2005), narrow pore throats ranging from 100 nm to 3 nm (Manohar, 1999) and negative surface charge that restricts the passage of hydrated anions. When OBM's interact with the rock the oil layer that surrounds the emulsified water acts as a perfect semi-permeable membrane (Lomba et al. 2000); the impact of the synergetic properties of the rock, the oil layer and the high capillary pressure of the mud makes the created membrane completely stops ionic flow. However, the measured membrane efficiency when OBM's interact with shales was not 100% as demonstrated by Al-Bazali (2005).
The membrane efficiency determination can be done through Pressure Transmission Test (PTT) or can be done thorugh Electrochemical Potential Test (EPT). The firs type of test was conducted by the authors Oort et al. (1996), Ewy & Stankovich (2000) and Schlemmer, Patel, Fiedheim, Young & Bloys (2003); this test consists of an hydraulic approximation to estimate membrane efficiency and is a direct measure of this property. In this test the shale samples are subjected to both hydraulic and osmotic gradients during exposure to drilling fluids. The pressure drop across the shale sample is measured and then converted to membrane efficiency. The problem with this procedure is than can be very time consuming and require shale core samples.
The second type of test is the Electrochemical Potential Test (EPT) that measures the voltaje drop across shale samples that are in contact with fluids of different salinities, the measured voltaje drop is used to indirectly calculate the membrane efficiency. It is a quick and easy method for obtaining the membrane efficiency of shale cuttings or cores, saving time and can be conducted at the Rig-Site.
The measurements of membrane efficiency done in PTT and EPT range the values of 0 to 50% for water base muds and ranges 50 to 80% for oil base muds. In the present study membrane efficiency mesures were not carried out, therefore we assume a optimistic value of membrane efficiency for Oil base muds of 80% in the following analyses.
MUD A: BALANCED ACTIVITY MUD
Normally, the Carbonera Formation is drilled using OBM with a volumetric water percentage between 40% and 20%. The strategy to minimize wellbore instability problems in this formation is to maintain the chemical balance between shale formation and non-aqueous drilling fluid. To do this, a balanced water activity Mud A was suggested. The mud activity in this case is equal to the activity of the formation. As a matter of fact, the osmotic pressure equals zero according to Equation 1 and there will be no water flow into or out of shale.
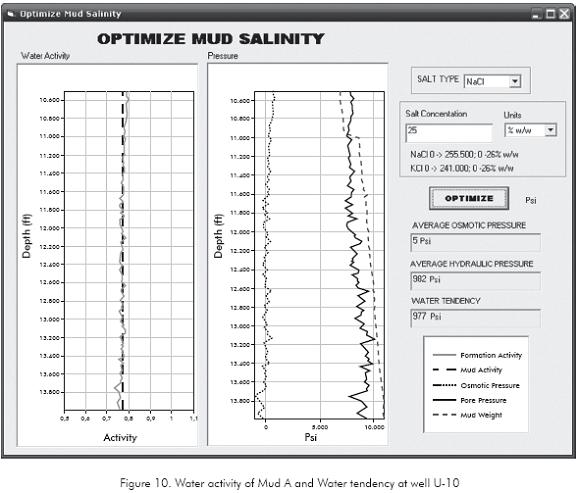
To determine the mud activity defining the balanced oil based mud activity for the depth interval 10 500- 14 000 ft in U-10 well, the geomechanical variables and laboratory test results were introduced in the Drilling Chemical Evaluation® software to define formation activity shown in Figure 10 (gray solid line). This figure shows the oil based mud activity proposed of 0,75 (dashed black line) which gives the average osmotic pressure equals to 5 psi. This little osmotic pressure indicates that in terms of chemical potential not will be a considerable water movement from mud to formation and viceversa. The Figure 10 also shows the osmotic pressure (dotted line in the pressure section), pore pressure (solid black line in the pressure section) and mud pressure (dashed gray line).
Once the osmotic pressure along the formation analyzed is determined, it's possible define the hydrated and dehydrated zones if only are take into account the chemical potential (osmotic forces) into the analysis (Figure 11).
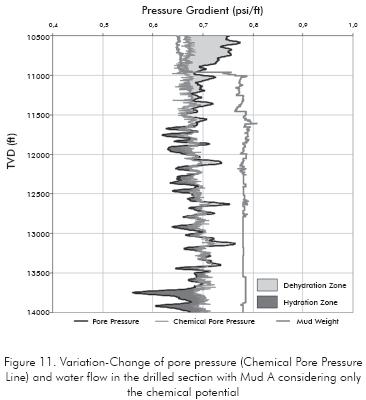
In the Figure 11 it's possible to see the hydrated zones (dark-gray area) and the dehydrated zones (gray area) along the drilled wellbore due to osmotic pressure effect over the in-situ pore pressure. Taking into account that the water flow due to a hydraulic gradient can often be several times smaller than the contribution of the chemical and thermal gradients (Ghassemi & Diek, 2003) the previous water flow is determinate without consider the mud pressure (hydraulic pressure) imposed over the formation. The chemical pore pressure line shows how the change in pore pressure is after the rock-fluid interaction.
To define if there are absorption or dehydration in a specific depth of the formation analyzed considering the mud pressure, is necessary define the concept of water tendency pressure as follows:
The water tendency pressure is the force that tries to move the water from o toward the formation depending of its sign. To explain the previous concept analyzes the case of Mud A where the average water tendency is 977 psi for Carbonera Formation, i.e., there is a tendency to hydrate the formation throughout the drilled section. The Mud A creates a chemical-mechanical condition at 12 500 ft show in Figure 12, where the hydrostatic mud pressure is 9758 psi, the pore pressure is 8500 psi and the osmotic pressure is -203 psi which indicates that at this depth the water tendency is 1461 psi.
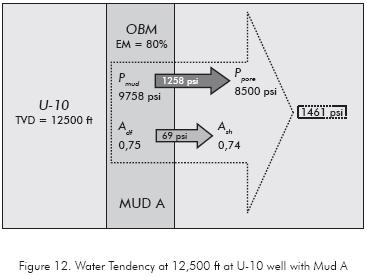
MUD B: LOW ACTIVITY MUD
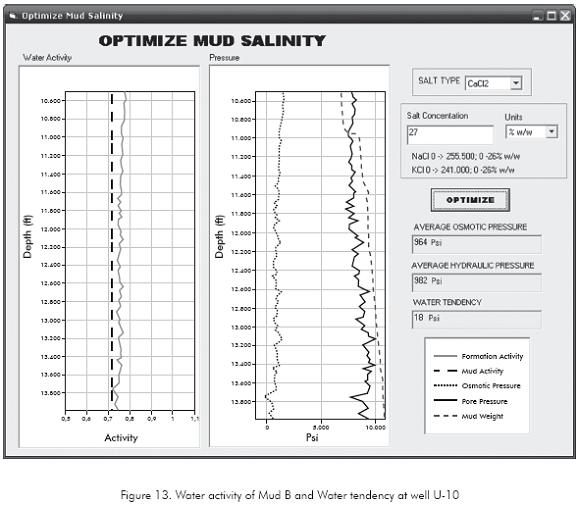
The intention with the appropriate drilling fluid formulation is not only to reduce the chemical effects of drilling fluid-rock interaction but also to use this chemical interaction to stabilize the formation. During the drilling of Carbonera Formation at U-10 well, the operation was always overbalanced with an average hydraulic pressure of 982 psi. The low water activity formulation of Mud B (Mud activity of 0,72) will produce an average effective osmotic pressure in the Carbonera Formation of 964 psi, as shown in Figure 13.
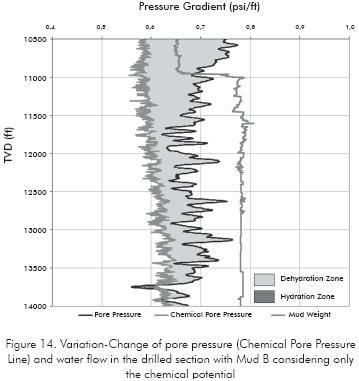
This osmotic pressure changes the pore pressure to chemical pore pressure show in Figure 14 which is more advantageous, because most of the drilled section is being dehydrated.
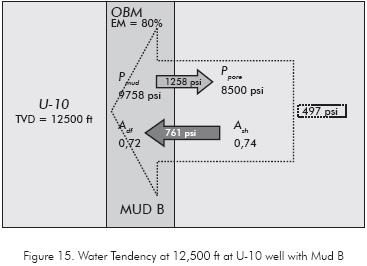
The osmotic pressure imposed balances the hydraulic pressure tendency to hydrate the Carbonera Formation reducing the average water tendency in this section to a value of 18 psi. The condition imposed by the Mud B at 12 500 ft. are improvement because the water tendency at this point is 761 psi which is lower than imposed by the Mud A. Figure 14 shows how this new water activity in the OBM affects the water tendency, however the hydration of the formation occurs.
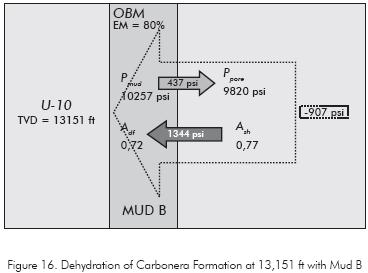
One particular condition takes place at 13 151 ft (Figure 16) where the water tendency is negative and the formation dehydration probably occurs.
DISCUSSION
The effective osmotic pressure evaluation in Carbonera Formation is successfully applied for the selection of OBM. According to Figures 11 and 14 the dehydration formation of some areas can be done through the use of Mud A and B. This decrease in water tendency to the formation significantly improves the borehole stability of the drilled section, especially with the Mud B which induces a chemical dehydration. This dehydration may be useful in preventing collapse, however, over-dehydration may destabilize wellbore stability and cause lost circulation.
It is important to point out that in the shaly formations the hydraulic flow is given in lower intensity that the osmotic flow, therefore in the present study the capacity of hydraulic flow was over estimated. Despite of this, to make the water tendency decreases to a minimum (to chemically stabilize the formation drilled) throughout the drilled formation is essential to determine an appropriate mud weight window that allows a near-balance drilling even in the shaly formations.
Throughout the determination of the chemical parameters, there are some variables that are not taking into account which increase the uncertainty of the calculations of the osmotic pressure, such as:
- - Formation temperature and in-situ pressure effects on the shale native water activity determined from the adsorption isotherm curve.
- Composition of formation pore fluid on the formation native water activity determined from the modified method of effective mean stress.
- Membrane efficiency determinate experimentally trough Pressure Transmission Test and how it varies with the concentration and salt type utilized in drilling fluid.
The last point is very important because the membrane efficiency controls the magnitude of the osmotic potential. A high chemical gradient imposed in the formation is useless if we don't have a highly efficient membrane. Factors like the cation-hydrated diameter (Al-Bazali et al. 2006), mineralogic composition of shale (Fritz, 1986) affect the membrane efficiency.
CONCLUSIONS
Based on the phenomenology of water flow (hydraulic and chemical potential gradient) the proposed methodology allows the selection of the mud activity in OBM's in Carbonera Formation and the following conclusions are derived from this study:
- The X-Ray diffraction test for the Carbonera Formation indicated that the 40% of bulk composition is clay and the predominant mineral is Kaolinite and Illite; the water content is 2,2% w/w and the formation activity of 0,78.
- Comprehensive integration of geomechanical data (stresses), pore pressure, formation temperature and core measurements (native water content and adsorption isotherm curve) are required to determinate the water activity of the Carbonera Formation through the method proposed by Zhang et al. (2006).
- The average osmotic pressures induced by the A and B muds were 5 psi and 964 psi for the Carbonera Formation. This induced osmotic pressure affect the pore pressure of the shaly formation and defines the new stability condition.
- The OBM balanced activity of Mud A stablish a dynamic equilibrium between the mud and pore fluid activity for the Carbonera Formation drilled, making the chemical water tendency (osmotic pressure) to average the value of zero.
- The stability condition can be improved over the Mud A condition by the use of OBM mud B activity. This Mud allows the osmotic pressure generated by the drilling-fluid rock interaction decreased the pore pressure in almost the whole drilled section. The high activity Mud B tends to reduce the pore pressure penetration of water within the formation and in some areas can cause dehydration which leads to an increase in the formation strength.
- A reduction of the mud activity (from A mud to B Mud) has a great effect in the chemical stability of the drilled area as long as the values of membrane efficiency are high. For that reason the membrane efficiency determination and how factors like pressure, temperature and fluids composition affecting its value are so important to determine whether the chemical effect (osmosis) can be effective method to stabilize the reactive shale formation.
- From analysis of the distribution of pore pressure presented in this article it is possible to carry out the geomecanichal model with the chemical-elastic coupling.
The integration of chemical interaction phenomenology into the wellbore stability modeling can be of great value during the drilling of challenging wells with narrow mud weight window and can provide increased understanding of drilling events and problems in shale formations.
ACKNOWLEDGEMENTS
The authors thank all members of wellbore stability group and to the Instituto Colombiano del Petróleo (ICP) Lab. team.
REFERENCES
Abbas, H., Shebatalhamd, A., Khan, M., Al-Shobaili, Y., Ansari, A., Ali, S., & Mehta, S. (2006). Wellbore in stability of shale formation; Zuluf Field, Saudi Arabia. Tech.l Symp. of Saudi Arabia, Dharhan, Saudi Arabia. SPE 106345. [ Links ]
Al-Bazali, T. M. (2005). Experimental study of the membrane behavior of shale during interaction with water-based and oil-based muds. Ph. D. Thesis, University of Texas at Austin, Texas, 272 pp. [ Links ]
Al-Bazali, M. T., Zhang, J., Chenevert, M. E., & Sharma, M. M. (2006). Factors controlling the membrane efficiency of shales when interacting with water-based and oil-based muds. International Oil & Gas Conference and Exhibition, Beijing, China. SPE 100735. [ Links ]
Ballard, T. J., Beare, S. P., & Lawless, T. A. (1992). Fundamentals of shale stabilization: water transport through shales. European Petroleum Conference, Cannes, France. SPE 24974. [ Links ]
Carminati, S., Del Gaudio, Zausa F., Brignoli, M. (1999). How do anions in water based muds affect shale stability? Int. Symp. on Oilfield Chemistry, Houston, Texas. SPE 50712. [ Links ]
Corzo, R. (2009). Modelamiento de la química del lodo sobre la resistencia a la compresión de rocas arcillosas. M.sc. Thesis, Scholl of Petroleum Engineering, Universidad Industrial de Santander, Bucaramanga, 280 pp. [ Links ]
Chenevert, M. E. (1970). Shale control with balanced-activity oil-continuous muds. J. Petrol. Tech., 1309-1316. [ Links ]
Chenevert, M. E., & Strassner, J. E. (1975). Temperature effects on water activities of argillaceous shales and oil mud systems. Fifteenth Oil and Gas Conference, Balatonfured, Hungary. [ Links ]
Da Fontoura, S. A. B., Rabe, C., & Lomba, R. F. T. (2002). Characterization of shales for Drilling Purposes. Rock Mechanics Conference, Irving, Texas. SPE 78218. [ Links ]
Ewy, R. T. & Stankovich, R. J. (2000). Pore pressure change due to shale-fluid interactions: Measurements under simulated wellbore conditions. Fourth North American Rock Mechanics Symp., Seattle, 147-154. [ Links ]
Fernández, W. A. (2008). Determinación de la eficiencia de membrana en rocas arcillosas a partir de la selectividad iónica para la obtención del gradiente de presión osmótico efectivo. B. Thesis. Universidad Industrial de Santander, Bucaramanga, 153. [ Links ]
Fonseca, C. F., & Chenevert, M. E. (1998). The effect of total stress and temperature on the water activity of shlaes. Int. J. Rock Mech. Min. Sci. 35: 546-547. [ Links ]
Fritz, S. J., & Marine, I. W. (1983). Experimental support for a predictive osmotic model of clay membranes. Geochimica et Cosmochimica Acta. 47: 1515- 1522. [ Links ]
Ghassemi, A., Diek, A., & Santos, H. (2001). Effects of ion diffusion and thermal osmosis on shale deterioration and borehole instability. AADE National Drilling Technical Conference, Omni, Houston. AADE01-NC-HO-40. [ Links ]
Ghassemi, A., & Diek, A. (2003). Linear chemo-poroelasticity for swelling shales: theory and application. J. Petrol. Sci. Eng. 38: 199-202. [ Links ]
Hale, A. H., Mody, F. K., & Sallsbury, D. P. (1993). The influence of chemical potential on wellbore stability. Soc. Petrol. Eng. Drilling and Completion. September. 207-216. [ Links ]
Lomba, R. F. T., Chenevert, M. E., & Sharma, M. M. (2000). The ion-selective membrane behavior of native shales. J. Petrol. Sci. Eng. 25: 9-23. [ Links ]
Manohar, L. (1999). Shale stability: drilling fluid interaction and shale strength. Latin American and Caribbean Petroleum Engineers, Caracas, Venezuela. SPE 54356. [ Links ]
Mody, F. F., Tare, U. A., Tan, C. P., Drummond C. J., & Wu B. (2002).Development of novel membrane efficient waterbased drilling fluids through fundamental understanding of osmotic membrane generation in shales. Annual Technical Conference and Exhibition, San Antonio, Texas. SPE 77447. [ Links ]
Oort, E. V., Hale, A. H., Mody, F. K., & Roy, S. (1996) Transport in shales and the design of improved water based shale drilling fluids. Soc. Petrol. Eng. Drilling & Completion, September. 137-146. [ Links ]
Reyes, R. A., & Vargas, J. G. (2005). Análisis del fenómeno de osmosis entre fluidos de perforación y formaciones arcillosas. B. Thesis. Universidad Industrial de Santander, Bucaramanga, 148 pp. [ Links ]
Santarelli, F. J., Chenevert, M. E., & Osisanya, S. O. (1992). On the Stability of Shales and its consequences in terms of swelling and wellbore stability. IADC/SPE Drilling Conference, New Orleans, Lousiana. SPE 23886. [ Links ]
Schlemmer, R., Patel, A., Friedheim, J., Young, S., & Bloys, J. B. (2003). Progression of water-based fluids based on amine chemistry: can the road lead to true oil mud replacement. National technology Conference, Houston, Texas. AADE-03-NTCE-36. [ Links ]
Tan, C. P., Drummond, C. J., Mody, F. K., & Tare, U. A. (2002). High membrane efficiency water-based drilling fluids: Alternatives to invert emulsion fluids for drilling troublesome shale formations. Asia Pacific Oil and Gas Conference and Exhibition, Melbourne, Australia. SPE 77865. [ Links ]
Yu, Mengjiao. (2002). Chemical and thermal effects on wellbore stability of shale formations. Ph. D. Thesis, University of Texas at Austin, Texas, 204 pp. [ Links ]
Zhang, J., Clark, D. E., Al-Bazali, T. M., Chenevert, M. E., Sharma, M. M., Rojas, J. C., & Ong, S. (2006). Ion movement and laboratory technique to control wellbore stability. Fluids Conference, Houston, Texas. AADE-06-DF-HO-37. [ Links ]
Zhang, J., Rojas, J. C., & Clark, D. E. (2008). Stressed-Shale Drilling Strategy - Water Activity Design Improves Drilling Performance, SPE Drilling and Completion, Vol. 23, 385-393. [ Links ]